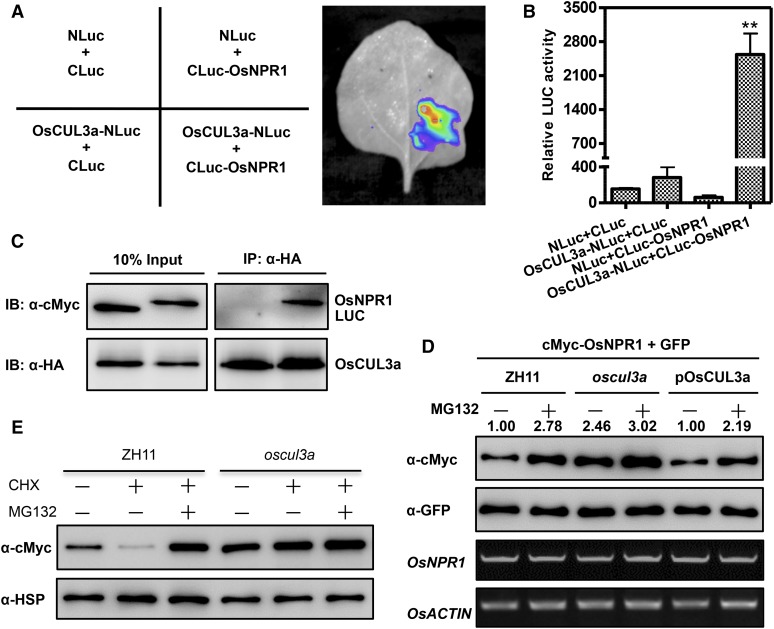
Figure 5.
OsCUL3a Interacts with OsNPR1 and Promotes Its Degradation in Vivo.
(A) OsCUL3a interacts with OsNPR1 as indicated by LCI assay. OsCUL3a-NLuc and CLuc-OsNPR1 were transiently expressed in N. benthamiana by coinfiltration; NLuc and CLuc were the negative controls. Luminescence was monitored with a low-light, cooled, CCD imaging apparatus at 3 dai.
(B) Quantification of the LUC activity in the leaves shown in (A). Error bars represent the se; n = 3 (t test between OsCUL3a-NLuc+CLuc-OsNPR1 and control group value; **P < 0.01).
(C) OsCUL3a interacts with OsNPR1 in a co-IP assay. Myc-tagged OsNPR1 or LUC was transiently expressed with HA-tagged OsCUL3a in rice protoplasts by cotransfection. Following total protein extraction, samples were immunoprecipitated with anti-HA antibody. Crude and immunoprecipitated proteins were analyzed with anti-HA or anti-Myc antibodies.
(D) OsCUL3a is required for OsNPR1 degradation in rice protoplasts. OsNPR1 was fused with the Myc tag and coexpressed with GFP in rice protoplasts isolated from ZH11, oscul3a, and pOsCUL3a plants. The transfected protoplasts were treated with or without MG132 for 20 h. Total protein was detected with anti-Myc and anti-GFP antibodies. The expression of OsNPR1 and OsACTIN was analyzed by RT-PCR. The relative abundance of OsNPR1 was calculated by comparing to GFP using ImageJ software. The OsNPR1/GFP relative quantity in ZH11 without MG132 was defined as 1.0 (lane 1).
(E) OsCUL3a-mediated degradation of OsNPR1 via the 26S proteasome. Myc-OsNPR1 was transiently expressed in rice protoplasts isolated from ZH11 and oscul3a plants with or without cycloheximide and MG132 treatments. Total protein was extracted at 48 h after transfection for immunoblotting analysis with anti-cMyc and anti-HSP antibodies.