Figure 1.
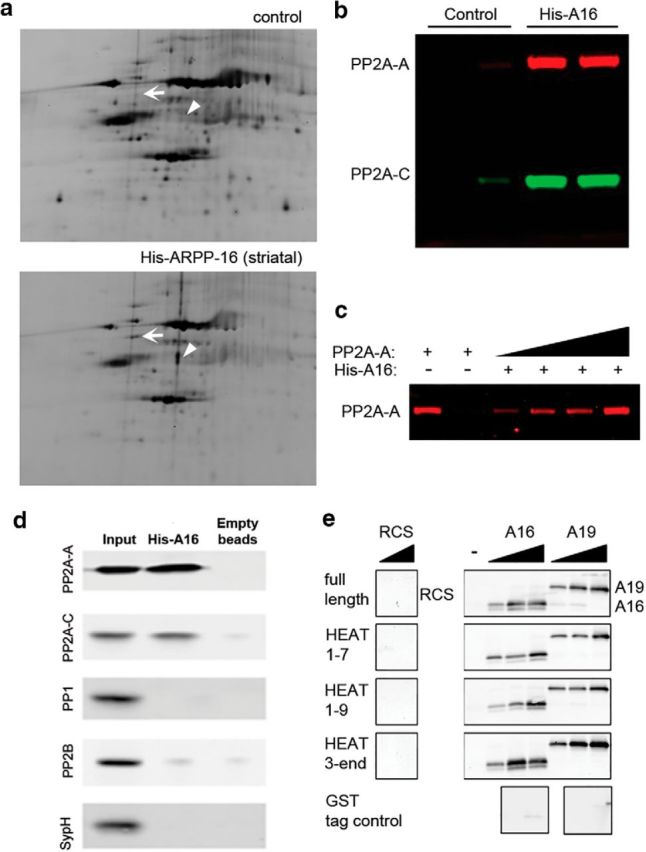
ARPP-16 interacts with the A subunit of PP2A. a, Rat brain S2 fractions were incubated with immobilized His-ARPP-16, and then eluted proteins were separated using DIGE. The striatal sample was labeled with Cy3, whereas the control sample (nonspecific binding of striatal samples to beads with no ARPP-16) was labeled with Cy2. Samples were mixed and analyzed by DIGE. White arrow indicates a spot corresponding to PP2A-A. Arrowhead indicates a spot corresponding to tubulin. b, Independent striatal S2 samples were incubated with control beads (Control) or beads with immobilized His-ARPP-16 (His-A16), and bound proteins were analyzed by SDS-PAGE and immunoblotting with antibodies to PP2A-A (red) and PP2A-C (green). c, Increasing amounts of recombinant, SEC-purified PP2A-A (10, 20, 40, or 80 ng) were incubated with immobilized His-ARPP-16 (50 μg) and bound protein analyzed by SDS-PAGE and immunoblotting with antibody to PP2A-A. The PP2A-A input (10 ng) is shown in lane 1, and the eluant from beads with no His-ARPP-16 that were incubated with PP2A-A (10 ng) is shown in lane 2. d, Striatal lysate fraction S2 was incubated with immobilized His-ARRP-16 (100 μg) or beads alone (empty beads, negative control). Eluted proteins were separated by SDS-PAGE and immunoblotted with antibody against PP2A-A, PP2A-C, PP1, PP2B, or synaptophysin. The lysate input (1%) is showed in lane 1; 20% of the eluates were analyzed in lanes 2 and 3. e, Full-length GST-PP2A-A, GST-tagged truncation mutants of PP2A-A that included HEAT repeats HEAT1–7, HEAT1–9, HEAT3-END, or a GST tag control (50 μg), were immobilized onto glutathione Sepharose 4 Fast Flow beads and incubated with increasing amounts of recombinant, SEC-purified His-ARPP-16 (A16, 10, 100, or 1000 nm) or SEC-purified His-ARPP-19 (A19, 10, 100, or 1000 nm). Recombinant RCS (100 or 1000 nm) was incubated with beads to determine nonspecific binding. Eluted samples were analyzed by SDS-PAGE and immunoblotted for ARPP-16, ARPP-19, or RCS.
