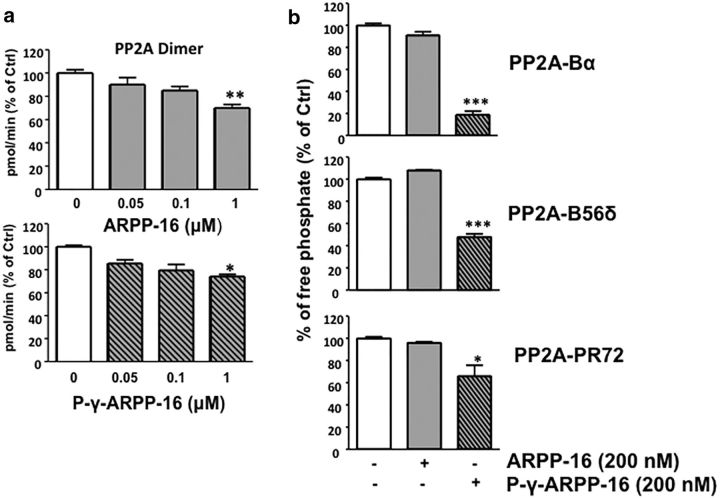
Figure 3.
ARPP-16 inhibits PP2A activity in vitro. a, Purified PP2A-AC dimer (0.01 U/μl, Millipore) was incubated with increasing concentration (0, 0.05, 0.1, and 1 μm) of recombinant, purified dephospho-ARPP-16 (top) or P-γ-Ser46-ARPP-16 (bottom) for 10 min at 37°C and phosphatase activity measured using 500 μm phosphopeptide as substrate. Phosphate release was detected using a malachite green assay with absorbance measured at 650 nm. b, Recombinant Flag-Bα, Flag-B56δ, or Flag-PR72 subunits were individually overexpressed in HEK293 cells, and PP2A heterotrimers were isolated by immunoprecipitation with anti-Flag antibody. PP2A heterotrimeric forms were incubated with 200 nm dephospho-ARPP-16 or P-γ-Ser46-ARPP-16 for 10 min at 37°C with [32P]-T75-DARPP-32 as substrate. 32P release was measured following TCA precipitation and scintillation counting. Results are all expressed as percentage changes with respect to PP2A alone (white bars). *p < 0.05 (Newman–Keuls test). **p < 0.01 (Newman–Keuls test). ***p < 0.001 (Newman–Keuls test). Error bars indicate SEM.