Abstract
Background
As downstream mediators of PI3K /PTEN /AKT /mTORC1 pathway, the AKT isoforms play critical roles in tumorgenesis. Although the pleiotropic effects of AKT1 in breast cancer have been reported, the genetic and epigenetic characteristics of AKT1 promoter region in breast cancer remains to be identified. In this study we aimed to investigate the promoter mutation spectrum, methylation and gene expression pattern of AKT1 and their relationship with breast cancer.
Methods
By using PCR target sequence enrichment and next-generation sequencing technology, we sequenced AKT1 promoter region in pairs of breast tumor and normal tissues from 95 unselected Chinese breast cancer patients. The methylation of the promoter region and the expression profile of AKT1 in the same cohort were detected with bisulfite next-generation sequencing and qPCR, respectively.
Results
We identified 28 somatic mutations in 23 of the 95 (24.2%) breast cancer samples. And 19 of the 28 mutations were located in transcription factor (TF) binding sites. In the 23 patients with somatic mutations, no significant change of methylation or expression was found comparing with other patients. AKT1 promoter region was significantly hypo-methylated in tumor compared with matched normal tissue (P = 0.0014) in the 95 patients. The expression of AKT1 was significantly suppressed in tumor tissue (P = 0.0375). In clinicopathological factor analysis, AKT1 showed significant hypo-methylation (P = 0.0249) and suppressed expression (P = 0.0375) in HER2 negative subtype. And a trend of decrease in expression level (P = 0.0624) of AKT1 in the ER negative subtype was observed, which is significantly decreased in basal-like breast tumor (P = 0.0328).
Conclusions
Hypo-methylation and suppressed expression of AKT1 was observed to be associated with breast cancer in our cohort. The methylation and expression of AKT1 were both significantly associated with HER2 status. The promoter mutation of AKT1 did not show significant association with its methylation and expression status. These results suggested that the promoter mutation, methylation and gene expression of AKT1 may play distinct roles in tumorgenesis of breast cancer and the integrated analysis of methylation and expression of AKT1 might serve as potential biomarkers for diagnosis and classification of breast cancer.
Introduction
Breast cancer is a leading cause of cancer-related death in women worldwide. According to GLOBOCAN 2012, there are estimated 1.67 million new breast cancer cases diagnosed with over half million deaths each year [1]. In recent years, breast cancer has become the most frequently diagnosed cancer in Chinese women, accounted for 12.2% of global cases and 9.6% of related deaths from breast cancer worldwide [2]. Breast cancer is a heterogeneous disease with distinct histopathological and molecular characteristics. According to the expression pattern of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2), breast cancer can be classified into four subtypes, including luminal A, luminal B, basal-like and HER2-positive [3]. Environment, genetics and immunological defects are major factors in the etiology of breast cancer [4]. Sporadic breast cancers account for approximately 90–95% of breast cancers, while familiar breast cancers account for the remaining 5–10% due to mutations in genes such as BRCA1/2 in breast cancer families [5].
Genome-wide studies of DNA sequence, copy number, gene structure and gene expression during the past decade have revealed remarkably diverse aberrations of many genes in breast tumors [6, 7]. AKT1 is a serine-threonine kinase gene, and involved in many processes including metabolism, proliferation, cell survival, growth and angiogenesis [8–10]. AKT1 is a downstream mediator of the PI3K / PTEN / AKT / mTORC1 pathway, which has been suggested to play crucial roles in the development of breast cancer [11, 12]. It has been reported that activation of AKT1 contributes to resistance to anti-proliferative signals and breast cancer progression [13, 14]. AKT1 mutations have been reported in 1.4% to 8% (average ~4%) invasive ductal breast carcinoma [15–18], and the oncogenic mutant loci E17K in AKT1 has been considered as a potential diagnosis biomarker of breast cancer [19].
The mutation and aberrant methylation in the promoter region of tumor suppressor genes, oncogenes, transcription factors and drug response genes could influence the gene expression and play important role in the tumorgenesis, tumor progression and response to treatment [20–22]. However, the AKT1 mutation and the methylation profile in its promoter region and their role in breast cancer are still not clear. In the present study, we analyzed the AKT1 promoter mutations with next generation sequencing in breast tumor and matched normal tissues from 95 unselected Chinese breast cancer patients. We also explored the methylation and expression alternation in this cohort with next-generation bisulfite sequencing and qPCR, respectively.
Material and methods
Patients and samples
Fresh breast tumor and matched adjacent normal tissues (located at least 2 cm away from the site of tumor tissue) from 95 unselected breast cancer patients were obtained from Xiangya Hospital, Central South University from year 2013 to 2015. The clinicopathological characteristics of patients were shown in Table 1. All breast specimens were reviewed by experienced pathologists. The breast cancer molecular subtypes were characterized based on the guideline of St. Gallen International Expert Consensus [3]. The study was approved by the Ethics Committee at Central South University, Changsha, China. All participants provided written informed consent.
Table 1. Clinicopathological characteristics of 95 breast cancer patients.
| Characteristics | Number of patients, n (%) | Number of patients with AKT1 promoter mutations, n (%)1 | P value2 | |
|---|---|---|---|---|
| Molecular subtype | Basal-like | 11 (11.58) | 3 (13.04) | 0.656 |
| HER2-enriched | 10 (10.53) | 2 (8.70) | ||
| Luminal A | 24 (25.26) | 5 (21.74) | ||
| Luminal B | 45 (47.37) | 13 (56.52) | ||
| Unknown | 5 (5.26) | 0 (0) | ||
| ER status | Positive (+) | 70 (73.68) | 18 (78.26) | 0.764 |
| Negative (-) | 25 (26.32) | 5 (21.74) | ||
| PR status | Positive (+) | 59 (62.11) | 14 (60.87) | 1 |
| Negative (-) | 36 (37.89) | 9 (39.13) | ||
| HER2 status | Positive (+) | 23 (24.21) | 5 (21.74) | 0.951 |
| Negative (-) | 60 (63.16) | 15 (65.22) | ||
| unknown | 12 (12.63) | 3 (13.04) | ||
| Lymph metastasis | Yes | 34 (35.79) | 8 (34.78) | 1 |
| No | 61 (64.21) | 15 (65.22) | ||
| Age | ≥50 | 50 (52.63) | 11 (47.83) | 0.772 |
| <50 | 45 (47.37) | 12 (52.17) | ||
Note:
1 the percentage was calculated in 23 patients with AKT1 promoter mutations;
2 P values were calculated between mutated and non-mutated patients using the Chi-square test.
Primer design
The 5’ promoter sequence for AKT1 was obtained from UCSC genome browser (http://genome.ucsc.edu/cgi-bin/hgGateway). For the mutation screening, we designed 4 pairs of primers covering the 5’ promoter region sequence of AKT1 up to 1000 bp using the online software Primer 3 (http://sourceforge.net/projects/primer3). For methylation analysis, the target-specific bisulfite sequencing primers (BSPs) were designed using the online design tool, Methprimer (http://www.urogene.org/methprimer/), with default parameters. The universal sequencing tags were added to the 5’-end of the forward and reverse primers by following the User Guide of Access Array™ System for Illumina Sequencing Systems (Fluidigm, South San Francisco, CA, USA). For expression analysis, the cDNA sequence was obtained from the Consensus CDS (http://www.ncbi.nlm.nih.gov/CCDS/CcdsBrowse/). Primers of GAPDH and AKT1 were designed cross exons using Primer 3 and the amplification efficiency was tested as approximate 100%. All the primers (S1 Table) were validated by conventional PCR and PCR products were confirmed for expected size on agarose gels.
Nucleic acid extraction
The genomic DNA was extracted from the paired tissues using the TIANamp Genomic DNA Kit (TianGen Biotech, Beijing, China) according to the manufacturer’s instruction. Total RNA extraction from the tissue samples was performed with TRIZOL-A reagent (TianGen Biotech, Beijing, China) according to the manufacturer’s instruction. The quality and quantity of all DNA and RNA samples were assessed on Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
DNA bisulfite conversion and RNA reverse transcription
Sodium bisulfite conversion of 500 ng genomic DNA was carried out using the EZ DNA Methylation-Lightning™ Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s instruction. All DNA samples after bisulfite conversion were quantified using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). For cDNA synthesis, 500 ng total RNA was reverse transcribed using a Revert Aid 1st Strand cDNA Synthesis Kit (Thermo Scientific, CA, USA) according to the manufacturer’s instruction.
Target sequence enrichment PCR and next generation sequencing
PCR was used for target enrichment to prepare sequencing libraries. PCR was performed in a Thermal Cycler (Bio-Rad T-100) with 10 μL reaction volume. PCR mix consisted of 1 μL (40 ng/μL) DNA or bisulfite converted DNA sample, 5.9 μL nuclease-free water, 1.8 μL Faststar High Fidelity reaction buffer (Roche, IN, USA), 0.2 μL dNTP, 0.1 μL (10 U/μL) DNA ploymerase (Roche, IN, USA) and 1 μL of each mutation sequencing primers or BSPs (2 μM). The PCR cycling conditions were: 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 30 s, 72°C for 1 min.
After PCR amplification, sample-specific 10-base barcodes and sequencing tags were added to each PCR product pool according to the User Guide of Access Array™ System for Illumina Sequencing Systems (Fluidigm, South San Francisco, CA, USA). Equal volume of each barcoded products were pooled into amplicon libraries and purified using Agencourt AMPure XP system (Beckman Coulter, CA, USA). The product size distribution was examined using Caliper LabChip GX (PerkinElmer, MA, USA). The purified libraries were quantified with Qubit® dsDNA HS Assay Kit (Life Technologies, CA, USA) and sequenced on a MiSeq sequencer using MiSeq Reagent Kit v2, 500 cycles (Illumina, CA, USA).
Sequencing data alignment for mutation detection and methylation analysis
After sequencing, the paired-end read data were demultiplexed by MiSeq Reporter (v.1.8.1) [23] according to sample specific barcodes with default parameters. After removing low quality reads, the sequences were aligned using BWA (v.0.7.10) with default parameters [24] to the UCSC human reference genome hg19. Germline mutations were called by the GATK Unified Genotyper with paired tumor and normal tissues [25], and somatic mutations were called by MuTect (v.1.1.4) [26]. We used the FIMO tool [27] to scan the promoter region of AKT1 for significant transcription factor binding sites (TFBSs) occurrences with a P value < le-4. The motif position weight matrices (PWMs) for TFBSs from HOCOMOCO (http://autosome.ru/HOCOMOCO/) were used as TFBSs motif input for FIMO. The methylation status and methylation level of each analyzed CpG-site were analyzed and returned from the BiQ Analyzer 3.0 software [28]. The methylation level of AKT1 was assigned by averaging the methylation level of all CpG sites located in the amplicon for each sample.
Gene expression analysis
To examine the gene expression of AKT1, real-time fluorescence quantitative polymerase chain reaction (qPCR) was performed in a CFX96™ Real-Time PCR Detection System (Bio-Rad, CA, USA). The gene expression primers for the AKT1 and the reference gene GAPDH were used for qPCR (S1 Table). All samples were assayed in triplicates. The qPCR mixture consisted of 2 μL of cDNA sample, 2 μL nuclease-free water, 5 μL 2 × SYBR Green PCR master mix (Roche, IN, USA), and 1 μL of each gene specific primer (2 μM). The PCR cycling conditions were: 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 5 s, 60°C for 30 s, and 72°C for 30 s, followed by dissociation curve analysis (65–95°C: increment 0.5°C for 5 s) to verify the amplification of a single product. The threshold cycle (Ct) value was determined using the default setting on the CFX Real-Time PCR Detection System. A mean of the Ct values for AKT1 and GAPDH were calculated for each sample, and expression level of AKT1 for each sample were determined using the delta Ct (dCt) method as follows: Mean Ct (AKT1)—Mean Ct (GAPDH), while a higher dCt value suggested lower expression level.
Statistical analysis
The paired Wilcoxon signed rank test was used to determine the difference of methylation and expression between paired tumor and normal tissues. The Spearman’s rank correlation coefficient test was applied to analyze correlation between methylation and expression for AKT1 in tumor and normal tissues. The Kruskal-Wallis rank sum test was used to examine the association of methylation or expression level with breast cancer subtypes. The Wilcoxon signed rank test was used to analyze the association of methylation or expression with other clinicopathological factors. Chi-square test was used for categorical data in mutation spectrum analysis. All statistical analysis was performed using R version 3.1.0 (http://www.cran.r-project.org). For all the above analysis, P value less than 0.05 was considered as statistically significant.
Results
AKT1 promoter mutation analysis
In the mutation analysis, we sequenced 1000 bp promoter region of AKT1 from 95 pairs of Chinese breast cancer tissues. We obtained high quality sequencing data with average gene read depth of 500 reads per sample. After applying the threshold at mutant allele fraction (AF) of >5%, totally 28 somatic mutant loci were detected in 23 of the 95 (24.2%) breast cancer patients, and most of the mutations were rare mutations. We predicted the TF binding site (TFBS) using the software FIMO. It revealed that in the 28 somatic mutations, 19 loci were located in TFBSs and 16 variants were predicted to result in loss or gain of TFBSs (Table 2). No germline mutation in the AKT1 promoter region was discovered in this cohort.
Table 2. The AKT1 methylation, expression and TFBS status of patients with promoter somatic mutations.
| Mutation Position (hg19) | Sample ID | Subtype | Mutant allele fraction (%) | Expression (dCt) | Methylation (%) | RefSeq_TF | Loss of TFBS | Gain of TFBS | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor | Normal | Tumor | Normal | |||||||
| chr14:g. 105262255G/A | S32_4 | Luminal A | 57 | 4.07 | 2.93 | 7.97 | 6.89 | . | . | |
| chr14:g. 105262441G/A | S8_3 | Luminal B | 16.87 | -0.85 | 3.59 | 9.32 | 11.76 | EGR1; KLF5; NR2C2; RREB1; SP1; SP2 | RREB1 | . |
| chr14:g. 105262373C/T | S1_4 | Luminal B | 13.6 | 4.05 | 4.27 | 6.82 | 7.21 | . | . | |
| chr14:g. 105262491G/A | S16_2 | Luminal B | 12.75 | 4.81 | 4.00 | 8.64 | 7.70 | EGR1; PAX5; PLAG1 | PLAG1 | ZNF263 |
| chr14:g. 105262266T/C | S14_3 | Luminal B | 12.62 | 4.60 | 4.10 | 5.02 | 7.15 | . | . | . |
| chr14:g. 105262526A/G | S2_4 | HER2 | 12.44 | 3.00 | 3.66 | 7.93 | 8.18 | . | . | HNF4A |
| chr14:g. 105262534T/G | S23_2 | Luminal B | 11.79 | 4.93 | 5.52 | 7.09 | 6.45 | . |
PLAG1; NR1H2; RXRA |
|
| chr14:g. 105262639C/T | S13_3 | Luminal B | 9.66 | 2.85 | 4.12 | 4.04 | 7.45 | REST; THAP1 | REST; THAP1 | . |
| chr14:g. 105262522C/T | S2_4 | HER2 | 8.52 | 3.00 | 3.66 | 7.93 | 8.18 | . | . | . |
| chr14:g. 105262269C/T | S10_3 | Luminal B | 8.32 | 2.11 | 3.91 | 8.14 | 7.00 | . | . | HOXA5; TLX1; NFIC |
| chr14:g. 105262286C/T | S10_3 | Luminal B | 8.09 | 2.11 | 3.91 | 8.14 | 7.00 | . | . | . |
| chr14:g. 105262333T/C | S23_2 | Luminal B | 7.79 | 4.93 | 5.52 | 7.09 | 6.45 | . | . | . |
| chr14:g. 105262931C/T | S6_4 | Luminal B | 7.59 | 3.89 | 3.52 | 9.38 | 8.95 | SMAD2; SMAD3; SMAD4 | SMAD2; SMAD3; SMAD4 | . |
| chr14:g. 105262991C/T | S8_2 | Luminal A | 7.5 | 3.23 | 3.19 | 6.15 | 8.65 | PLAG1 | PLAG1 | . |
| chr14:g. 105262531T/C | S42_4 | Luminal A | 6.86 | 2.55 | 4.00 | 6.59 | 11.18 | . | . | . |
| chr14:g. 105262293G/A | S34_4 | Luminal B | 6.69 | 4.46 | 3.34 | 4.97 | 8.14 | . | . | RREB1 |
| chr14:g. 105262937C/T | S38_4 | Basal-like | 6.45 | 2.06 | 3.88 | 9.80 | 8.06 | INSM1 | INSM1 | EWSR1-FLI1 |
| chr14:g. 105262848A/G | S2_4 | HER2 | 6.30 | 3.00 | 3.66 | 7.93 | 8.18 | CTCF | . | . |
| chr14:g. 105262438C/A | S45_4 | Luminal A | 6.29 | 4.53 | 3.41 | 9.53 | 6.43 | EGR1; KLF5; NR2C2; RREB1; SP1; SP2 | NR2C2 | E2F4; MZF1_5–13 |
| chr14:g. 105262295C/T | S21_4 | Luminal B | 6.28 | 4.40 | 4.56 | 7.50 | 6.65 | . | . | . |
| chr14:g. 105262829C/A | S10_2 | HER2 | 6.25 | 4.32 | 5.14 | 8.63 | 5.68 | HNF4A; HNF4G | HNF4G | . |
| chr14:g. 105262338A/G | S26_4 | Luminal B | 6.18 | 4.38 | 3.69 | 6.72 | 8.34 | . | . | . |
| chr14:g. 105262863C/A | S2_4 | HER2 | 6.09 | 3.00 | 3.66 | 7.93 | 8.18 | BATF; JUN; JUN (var.2) | JUN (var.2) | |
| chr14:g. 105262419T/C | S35_4 | Luminal B | 5.64 | 3.22 | 4.83 | 10.60 | 7.85 | EGR1 | . | SP2 |
| chr14:g. 105262542G/A | S37_4 | Basal-like | 5.52 | 2.98 | 4.31 | 11.37 | 7.17 | EBF1; ZNF263 | EBF1 | . |
| chr14:g. 105262975C/T | S31_4 | Basal-like | 5.47 | 6.19 | 4.68 | 9.75 | 8.62 | EGR1; KLF5; SP1; SP2 | SP1; KLF5; SP2; EGR1 | . |
| chr14:g. 105262503G/A | S27_4 | Luminal A | 5.46 | 3.67 | 3.91 | 5.35 | 10.23 | PAX5 | . | . |
| chr14:g. 105262690G/A | S17_3 | Luminal B | 5.42 | 5.78 | 3.66 | 7.64 | 10.35 | ZNF263 | . | . |
The clinicopathological characteristics of 23 patients with AKT1 promoter mutation were shown in Table 1. Chi-square test was used to detect if mutation in promoter region is associated with clinical characteristics. No significant association was found between the mutations and these clinicopathological factors.
AKT1 promoter methylation and gene expression analysis
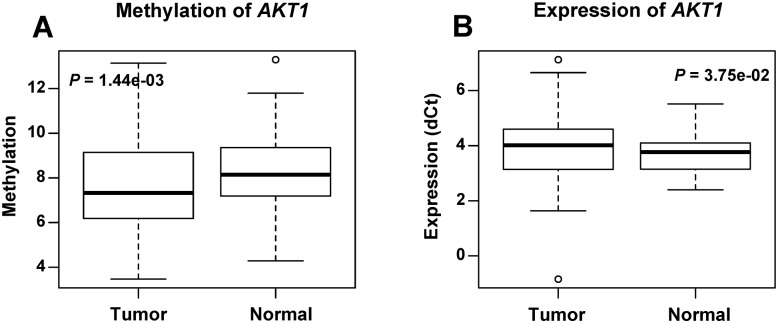
Sequencing of bisulfite-converted genomic DNAs revealed that the AKT1 promoter region were hypo-methylated in breast tumor tissues compared with the matched adjacent normal tissues (Fig 1). Average methylation level of the 10 CpG sites within 172 bp of AKT1 promoter region showed significant difference between the 95 tumor (7.49%) and matched normal tissues (8.35%) (P = 0.00144). The AKT1 expression analysis showed that average gene expression level (dCt) of AKT1 in tumor is lower than normal tissues. A significant difference (P = 0.0375) between the tumor and normal tissues was observed (Fig 1). These results showed significant lower methylation level and significant lower gene expression level (high dCt) in tumor tissues than normal tissues, but no significant cis correlation was found between the methylation and the expression level (P = 0.160, R2 = 0.010) using Spearman’s rank correlation analysis (S1 Fig).
Fig 1. The methylation and expression level of AKT1 in tumor and normal tissues from 95 breast cancer patients.
Boxplots show the average methylation (A) and expression (B) of AKT1 in breast tumor and normal tissues, and P values were calculated using the paired Wilcoxon signed rank test.
Correlation of the methylation and expression level of AKT1 gene with patient clinicopathological characteristics
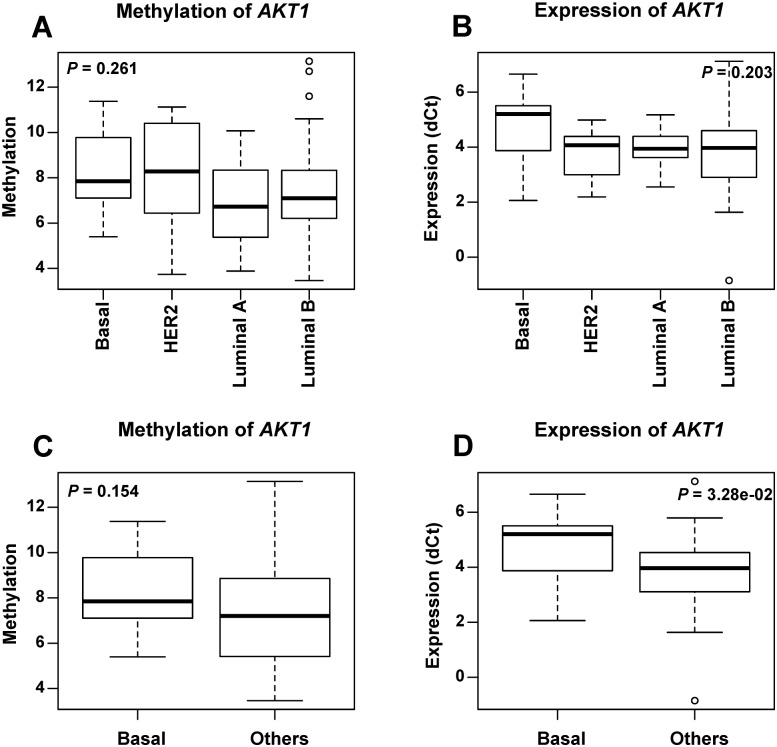
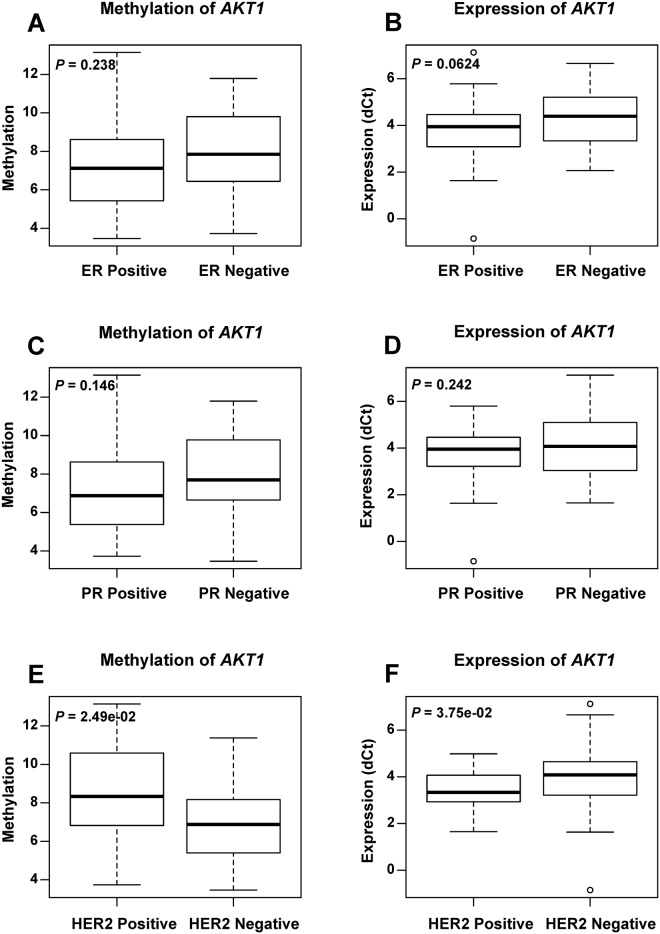
We analyzed the AKT1 methylation and expression level according to the clinicopathological characteristics of the breast cancer patients (S2 Table). No significant difference either in the methylation or in gene expression was observed among four breast cancer subtypes. However, it was obvious that the basal-like breast cancer subgroup showed the lowest mean expression level of AKT1 (Fig 2). In the comparison of AKT1 methylation and expression between basal-like tumor and other subtypes, it revealed significant lower AKT1 expression (P = 0.0328) in basal-like breast cancer than other subtypes (Fig 2). The analysis of the methylation and expression according to ER, PR and HER2 (Fig 3) status showed that the methylation (P = 0.0249) and expression (P = 0.0375) of AKT1 were significantly associated with HER2 status. The hypo-methylation and suppressed expression of AKT1 were associated with HER2 negative tumors. The expression level in ER negative breast cancer was lower than that in the ER positive subtypes, although it was not significantly different (P = 0.0624). The low expression level of AKT1 in ER negative and HER2 negative tumor is consistent with low expression in basal-like tumor which is ER and HER2 negative. In addition, no significant difference was observed according to the status of age and lymph metastasis (data not shown).
Fig 2. The methylation and expression level of AKT1 in breast cancer subtypes.
Boxplots show the average methylation (A) and expression (B) level of AKT1 in four different breast cancer subtypes, as well as methylation (C) and expression (D) in basal-like and non-basal-like breast tumor tissues. P values were calculated using the Kruskal—Wallis rank sum test for breast cancer subtypes and the Wilcoxon signed rank test for basal-like and non-basal-like breast tumor tissues.
Fig 3. The methylation and expression level of AKT1 in breast cancer patients according to ER/PR/HER2 status.
Boxplots show the average methylation (A, C, E) and expression (B, D, F) level of AKT1 between ER-positive and ER-negative subgroups, between PR-positive and PR-negative subgroups, as well as between HER2-positive and HER2-negative subgroups. P values were calculated using the Wilcoxon signed rank test.
AKT1 methylation and expression analysis in patients with promoter mutations
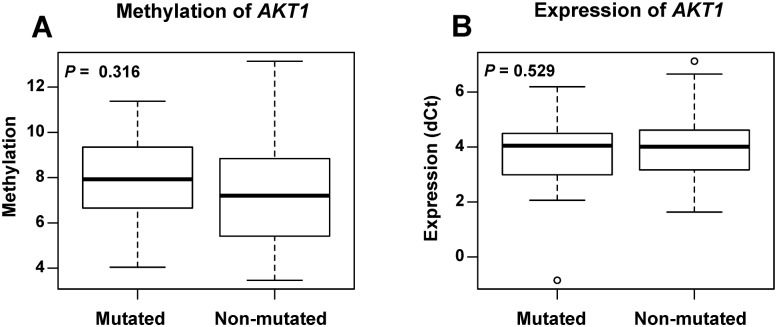
Totally, 23 tumor tissue samples carried 28 somatic mutations (Table 2) in the promoter region of AKT1. We compared the methylation level between mutated and non-mutated tumor samples. No significant difference of methylation was found between these two groups of tumors (P = 0.316) (Fig 4). In addition, we looked at the expression level of AKT1 in the samples with mutations. The expression was depressed in 9 tumors, and elevated in other 14 tumors (Table 2). However, no significant expression difference was found between mutated and non-mutated tumors (P = 0.529) (Fig 4). We did not observe any significant association of AKT1 promoter mutation with its methylation and expression.
Fig 4. The methylation and expression level of AKT1 in breast cancer patients with AKT1 mutations.
Boxplots show the average methylation (A) and expression (B) level of AKT1 in patient tumor tissues with or without AKT1 promoter mutations. P values were calculated using the Wilcoxon signed rank test.
Discussion
Aberrations of phosphatidylinositol 3-kinase PI3K/AKT pathway were frequently observed in cancer. Mutations and aberrant methylation of members in PI3K/AKT pathway play critical roles to regulate gene expression involved in tumorgenesis and metastasis [8]. This pathway therefore emerges as one of the promising targets of anticancer drugs in the near future. In this study, we investigated the AKT1 promoter mutation spectrum, methylation pattern and their potential role on its expression. Hypo-methylation and decreased expression of AKT1 were observed significantly associated with breast cancer in this study.
Since the E17K mutation of AKT1 was firstly identified as a potential biomarker in breast cancer, the coding region of AKT1 has become the hotspot of mutation detection [18]. However, the mutation spectrum in promoter region of AKT1 in breast cancer is still unclear. In the mutation analysis, we found somatic mutation (AF>5%) in 24.2% of breast cancer patients (23 in 95) indicating that AKT1 promoter mutation may be a frequent event in breast cancer. And mutation loci distributed in all subtypes of breast cancer. These results were different from the previous meta analysis on AKT1 mutation, in which only 3.8% AKT1 mutation was detected in breast cancer patient and restricted to hormone receptor—positive cancers [18, 29, 30]. It could be explained by the following two aspects. Firstly, the breast cancer gene mutation pattern may be different in races. Secondly, the reported sequence target was the exon regions, while our target was the promoter region of AKT1. It implied that the promoter region may be the mutation hotspot of AKT1 in Chinese breast cancer patients. Wang et al [31] reported three SNPs (rs2494750 G>C, rs2494752 A>G, and rs10138227 C>T) in AKT1 promoter region in gastric adenocarcinoma. AKT1 rs2494750 G>C located in our target sequence, which we also observed in this study (data not shown). In addition, we analyzed the TFBS change and the corresponding expression alternation in the 23 tumors harbored somatic mutations. AKT1 expression was elevated in 14 and reduced in 9 breast tumors. In general, we did not find AKT1 promoter mutations significantly associated with its gene expression in the 95 breast cancer patients. These observations still need further validation in large cohorts.
Increasing evidence indicated that tumorgenesis depends on not only the acquisition of genetic alterations, but also epigenetic perturbations, which adds an important layer of transcriptional control to the cancer genome. It has been shown that DNA methylation at gene promoter regions plays a critical role in maintaining silencing of tumor suppressor genes in tumors, including breast cancer. The promoter hyper/hypo-methylation is linked and perhaps directly contributes to tumorgenesis, invasion, metastasis, and chemotherapeutic resistance [32]. Mutation and increased phosphorylation of AKT1 were identified in different types of cancers, including melanoma, breast, esophageal, colorectal, endometrial, ovarian, and non-small cell lung cancers [33]. In the present study, AKT1 was significantly hypo-methylated and less expressed in the breast tumor compared with the matched normal tissue, but no significant cis correlation was found between methylation and expression. Similar situation was observed in several studies, which supported the notion that methylation is sufficient but not necessary for their inactivation of gene expression [34, 35]. We analyzed and checked the methylation and expression data of AKT1 in TCGA from MethHC database (http://methhc.mbc.nctu.edu.tw/php/index.php). AKT1 also showed significant hypo-methylation in breast tumor tissues in TCGA, which is consistent with our results. AKT1 showed significant difference of expression between tumor and normal tissue in TCGA but with higher expression in tumor. The discrepancy observed here is most likely related to the differences in detection methods, stage or type of breast tumor, and even the differences in race or ethnicity [36].
In the subtype analysis, we found that AKT1 expression was suppressed in basal-like tumors and HER2 negative tumors, comparing with that in Lumina A and Lumina B subtypes. Previous publication has demonstrated that constitutively activated AKT1 expression inhibited the basal-like breast cancer cell line MDA-MB231 cells proliferation [37]. In addition, over-expressed pAKT1 in HER2 positive breast cancer was associated with poor prognosis [38, 39], indicating a post-translational modification mechanism for AKT1 in breast cancer. Our observation suggested that AKT1 expression aberrations likely play distinct role in the pathogenesis of different breast cancer subtypes [18]. In the comparison of the methylation level between the mutated and non-mutated specimens, no significant difference was found. This suggested that the AKT1 promoter mutation was not an influence factor on its methylation and they may play distinct role in tumorigeneis.
In summary, AKT1 promoter mutation and methylation alternation were observed commonly in our cohort of Chinese breast cancer patients. The promoter hypo-methylation and decreased gene expression were associated with breast tumor. The AKT1 promoter mutation, methylation and expression may play distinct roles in breast cancer and could be potential biomarkers for breast cancer diagnosis and classification. The decrease of AKT1 expression was observed in basal-like and HER2 negative breast tumor, which could benefit for diagnosis and targeted therapy of basal-like breast cancer based on subtype. The results of present study were found in a relative small cohort, which may need further validation. Consequent confirmation of our discoveries in a larger breast cancer cohort might lead to a better understanding of breast cancer pathogenesis and benefit breast cancer early detection and classification.
Supporting information
Spearman’s rank correlation test was used for the cis correlation analysis between methylation and expression. Normal tissue in green and tumor in red.
(TIF)
(DOCX)
(XLSX)
Acknowledgments
This work was supported in part by grants from the Natural Science Foundation of China (No. 81272296 and 81372228), and the Major Special Projects of the Science and Technology Bureau of Changsha, China (No. K1204017-31 and K1306011-31).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by grants from the Natural Science Foundation of China (No. 81272296 and 81372228), and the Major Special Projects of the Science and Technology Bureau of Changsha, China (No. K1204017-31 and K1306011-31).
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. The Lancet Oncology. 2014;15(7):e279–89. 10.1016/S1470-2045(13)70567-9 [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24(9):2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nature reviews Cancer. 2004;4(11):850–60. 10.1038/nrc1476 [DOI] [PubMed] [Google Scholar]
- 5.Garber JE, Offit K. Hereditary cancer predisposition syndromes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(2):276–92. [DOI] [PubMed] [Google Scholar]
- 6.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400–4. 10.1038/nature11017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray J, Druker B. Genomics: the breast cancer landscape. Nature. 2012;486(7403):328–9. 10.1038/486328a [DOI] [PubMed] [Google Scholar]
- 8.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74. 10.1016/j.cell.2007.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoeli-Lerner M, Toker A. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell cycle. 2006;5(6):603–5. 10.4161/cc.5.6.2561 [DOI] [PubMed] [Google Scholar]
- 10.Chin YR, Toker A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cellular signalling. 2009;21(4):470–6. 10.1016/j.cellsig.2008.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis NM, Sokolosky M, Stadelman K, Abrams SL, Libra M, Candido S, et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget. 2014;5(13):4603–50. 10.18632/oncotarget.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paplomata E, O'Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Therapeutic advances in medical oncology. 2014;6(4):154–66. 10.1177/1758834014530023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nature medicine. 2002;8(10):1153–60. 10.1038/nm761 [DOI] [PubMed] [Google Scholar]
- 14.Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Molecular cell. 2010;38(3):333–44. 10.1016/j.molcel.2010.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleeker FE, Felicioni L, Buttitta F, Lamba S, Cardone L, Rodolfo M, et al. AKT1(E17K) in human solid tumours. Oncogene. 2008;27(42):5648–50. 10.1038/onc.2008.170 [DOI] [PubMed] [Google Scholar]
- 16.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486(7403):405–9. 10.1038/nature11154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flatley E, Ang D, Warrick A, Beadling C, Corless CL, Troxell ML. PIK3CA-AKT pathway mutations in micropapillary breast carcinoma. Human pathology. 2013;44(7):1320–7. 10.1016/j.humpath.2012.10.018 [DOI] [PubMed] [Google Scholar]
- 18.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer research. 2008;68(15):6084–91. 10.1158/0008-5472.CAN-07-6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolph M, Anzeneder T, Schulz A, Beckmann G, Byrne AT, Jeffers M, et al. AKT1 (E17K) mutation profiling in breast cancer: prevalence, concurrent oncogenic alterations, and blood-based detection. BMC cancer. 2016;16:622 10.1186/s12885-016-2626-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahat B, Thakur S, Hamid A, Bagga R, Kaur J. Association of aberrant methylation at promoter regions of tumor suppressor genes with placental pathologies. Epigenomics. 2016;8(6):767–87. 10.2217/epi.16.7 [DOI] [PubMed] [Google Scholar]
- 21.Colebatch AJ, Di Stefano L, Wong SQ, Hannan RD, Waring PM, Dobrovic A, et al. Clustered somatic mutations are frequent in transcription factor binding motifs within proximal promoter regions in melanoma and other cutaneous malignancies. Oncotarget. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostroma II, Gritsaev SV, Sidorova Zh Y, Tiranova SA, Svitina SP, Drizhun YS, et al. [Aberrant methylation of the promoter regions of the SOX7 and p15INK4b genes and Wnt signaling pathway antagonists in patients with acute myeloid leukemias]. Terapevticheskii arkhiv. 2016;88(7):31–6. [DOI] [PubMed] [Google Scholar]
- 23.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome research. 2010;20(9):1297–303. 10.1101/gr.107524.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nature biotechnology. 2013;31(3):213–9. 10.1038/nbt.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant CE, Bailey TL, Noble WS. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27(7):1017–8. 10.1093/bioinformatics/btr064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bock C, Reither S, Mikeska T, Paulsen M, Walter J, Lengauer T. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 2005;21(21):4067–8. 10.1093/bioinformatics/bti652 [DOI] [PubMed] [Google Scholar]
- 29.Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast cancer research: BCR. 2011;13(6):224 10.1186/bcr3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troxell ML. PIK3CA/AKT1 Mutations in Breast Carcinoma: a Comprehensive Review of Experimental and Clinical Studies. J Clinic Experiment Pathol 2012;S1(002). [Google Scholar]
- 31.Wang MY, He J, Zhu ML, Teng XY, Li QX, Sun MH, et al. A Functional Polymorphism (rs2494752) in the AKT1 Promoter Region and Gastric Adenocarcinoma Risk in an Eastern Chinese Population. Scientific reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92. 10.1016/j.cell.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishizawa D, Kasai S, Hasegawa J, Sato N, Tanioka F, Sugimura H, et al. Association between AKT1 Gene Polymorphism rs2498794 and Smoking-Related Traits with reference to Cancer Susceptibility. BioMed research international. 2015;2015:316829 10.1155/2015/316829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Heng J, Yan J, Guo X, Tang L, Chen M, et al. Integrated analysis of gene expression and methylation profiles of 48 candidate genes in breast cancer patients. Breast cancer research and treatment. 2016. [DOI] [PubMed] [Google Scholar]
- 35.Hon GC, Hawkins RD, Caballero OL, Lo C, Lister R, Pelizzola M, et al. Global DNA hypomethylation coupled to repressive chromatin domain formation and gene silencing in breast cancer. Genome research. 2012;22(2):246–58. 10.1101/gr.125872.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Heng J, Yan J, Guo X, Tang L, Chen M, et al. Integrated analysis of gene expression and methylation profiles of 48 candidate genes in breast cancer patients. Breast cancer research and treatment. 2016;160(2):371–83. 10.1007/s10549-016-4004-8 [DOI] [PubMed] [Google Scholar]
- 37.Yang W, Ju JH, Lee KM, Shin I. Akt isoform-specific inhibition of MDA-MB-231 cell proliferation. Cellular signalling. 2011;23(1):19–26. 10.1016/j.cellsig.2010.07.016 [DOI] [PubMed] [Google Scholar]
- 38.Lin Fde M, Bacchi CE, Baracat EC, Carvalho FM. Loss of PTEN expression and AKT activation in HER2-positive breast carcinomas. Revista brasileira de ginecologia e obstetricia: revista da Federacao Brasileira das Sociedades de Ginecologia e Obstetricia. 2014;36(8):340–6. [PubMed] [Google Scholar]
- 39.Tokunaga E, Kimura Y, Oki E, Ueda N, Futatsugi M, Mashino K, et al. Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients. International journal of cancer. 2006;118(2):284–9. 10.1002/ijc.21358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spearman’s rank correlation test was used for the cis correlation analysis between methylation and expression. Normal tissue in green and tumor in red.
(TIF)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.