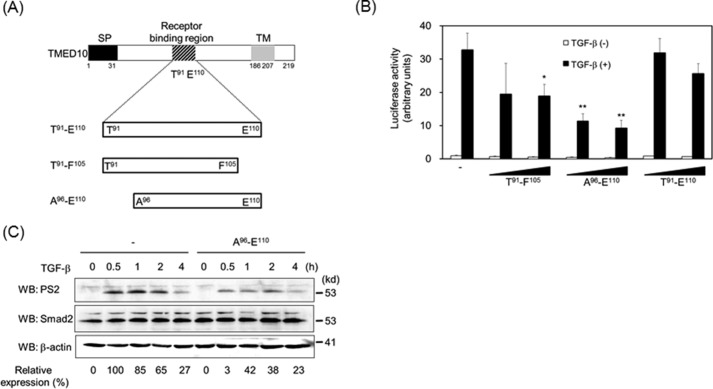
FIGURE 6.
Fifteen-amino acid-long peptide as a unique inhibitor of TGF-β signaling. A, illustration of the synthesized peptides. SP, signal peptide; TM, transmembrane. B, inhibition of TGF-β-driven reporter activity by the synthesized peptides. The experiments were performed as described in Fig. 2A except for the addition of 200 nm or 1 μm peptide 2 h prior to TGF-β (5 ng/ml) stimulation. Significant differences from the control in the presence of TGF-β are indicated with asterisks. C, inhibition of TGF-β-induced Smad2 phosphorylation by peptides from Ala96 to Glu110. Two hours after the addition of 1 μm peptide, HepG2 cells were stimulated with 0.5 ng/ml TGF-β for the indicated times. Western blotting analyses (WB) were performed as described in Fig. 2F. The top, middle, and bottom panels show the expressions of phosphorylated Smad2, Smad2, and β-actin, respectively. The expression of phosphorylated Smad2 upon TGF-β stimulation was normalized using the intensity of the band corresponding to Smad2. Relative expression was calculated relative to the value without peptide in the absence of TGF-β. Probability values below 0.01 and 0.001 were considered significant: **, p < 0.01,***, p < 0.001.