Abstract
The pathogenesis of multiple myeloma (MM) has not yet been fully elucidated. Our microarray analysis and immunohistochemistry revealed significant up-regulation of growth arrest-specific gene 6 (Gas6), a vitamin K-dependent protein with a structural homology with protein S, in bone marrow (BM) cells of MM patients. ELISA showed that the serum levels of soluble Gas6 were significantly increased in the MM patients when compared with healthy controls. Gas6 was overexpressed in the human CD138-positive MM cell line RPMI-8226. Exogenous Gas6 suppressed apoptosis induced by serum deprivation and enhanced cell proliferation of the MM cells. The conditional medium from the human BM stromal cell line HS-5 induced cell proliferation and anti-apoptosis of the MM cells with extracellular signal-regulated kinase, Akt, and nuclear factor-κB phosphorylation, which were reversed by the neutralizing antibody to Gas6 or IL-6. The TAM family receptor Mer, which has been identified as a Gas6 receptor, was overexpressed in BM cells of MM patients. The knockdown of Mer by siRNA inhibited cell proliferation, anti-apoptosis, and up-regulation of intercellular cell adhesion molecule-1 (ICAM-1) in MM cells stimulated by an HS-5 cell-conditioned medium. Furthermore, the Gas6-neutralizing antibody reduced the up-regulation of IL-6 and ICAM-1 induced by a HS-5 cell-conditioned medium in MM cells. The present study provides new evidence that autocrine and paracrine stimulation of Gas6 in concert with IL-6 contributes to the pathogenesis of MM, suggesting that Gas6-Mer-related signaling pathways may be a promising novel target for treating MM.
Keywords: apoptosis, bone marrow, cell proliferation, interleukin 6 (IL-6), multiple myeloma, stromal cell
Introduction
Multiple myeloma (MM)3 is a hematologic neoplasm characterized by proliferation of malignant plasma cells in part due to autocrine and paracrine loops involving the surrounding microenvironment; however, the mechanism of its disease progression remains largely unknown (1–3). The vitamin K-dependent protein growth arrest-specific gene 6 (Gas6), which is structurally homologous to anticoagulant protein S, is a cofactor for protein C that works in concert as a natural anticoagulant system (4–6). Gas6 is the ligand that binds to Tyro3, Axl, and Mer (TAM) receptors, which belong to a family of receptor tyrosine kinases (6, 7). Moreover, Gas6 is expressed not only in normal blood and bone marrow (BM) cells but also in several cancer cells (8–14). Tumorigenic processes are involved in Gas6/TAM signaling, which may be a prognostic and predictive biomarker, as well as in identifying potential therapeutic targets (10, 11). The Gas6/Axl axis in the BM environment regulates cell invasion, proliferation, and survival of prostate cancer cells, resulting in metastasis dormancy in the BM niche (14, 15). Some investigators have reported that Gas6-related signaling pathways may be critical for the progression mechanisms in hematological malignancies (16–18). Gas6 receptor Mer is aberrantly overexpressed in a majority of primary acute myeloid leukemia (AML) patient blasts and contributes to leukemogenesis in AML (16). Paracrine cross-talk between AML cells and bone marrow stroma promotes Gas6 secretion, which fosters AML cell growth and chemoresistance (17, 18). Furthermore, paracrine action of Gas6 secreted by osteoblasts activates the mitogen-activated protein kinase signaling pathway in Mer-overexpressed (1, 19) (q23;p13) translocation (E2A/PBX1)-positive acute lymphoblastic leukemia cells, resulting in cell proliferation and anti-apoptosis (18). Gas6/Mer autocrine signaling axis promotes the proliferation and survival of MM cells, suggesting that this signaling pathway represents a novel candidate for therapeutic intervention in this incurable malignancy (3).
In the present study, we hypothesized that Gas6 may contribute to anti-apoptosis and cell proliferation of MM cells, which simultaneously enhance the generation of Gas6 in concert with interleukin-6 (IL-6) from both MM cells and BM stromal cells (BMSCs) through paracrine and autocrine mechanisms.
Results
Gas6 Overexpression in BM and Serum in Patients with MM
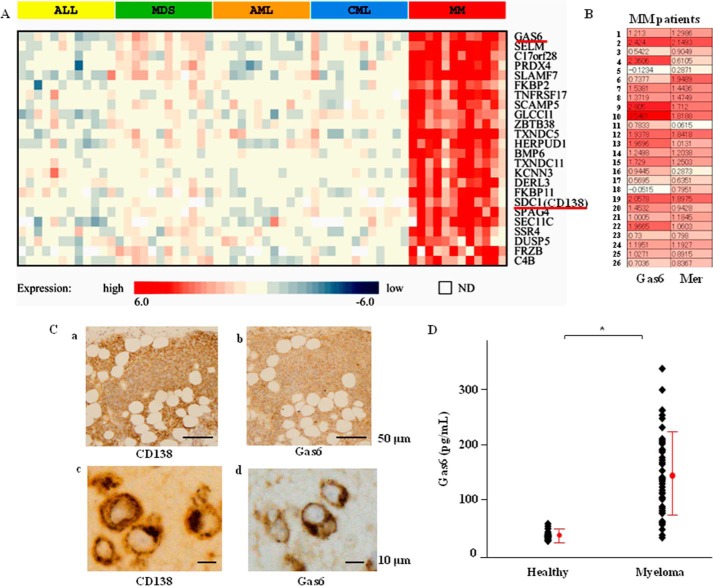
Blood or BM was obtained from 12 patients, each with acute lymphoid leukemia, myelodysplastic syndrome, AML, chronic myeloid leukemia, and MM, and the gene expression profiles of these samples were obtained. To identify the genes that were specifically expressed at high levels in MM, a multiple-group comparison was performed. Significant differences (p < 0.01) were observed between MM and other hematological malignancies, wherein genes that were expressed at a higher ratio in MM (a mean of 2.5 or greater) were identified (Fig. 1A). The heat map was created by calculating the mean ratio of expression in all tissue samples except for those of MM and then subtracting that ratio from each of the other ratios. In other words, the heat map indicates the ratio of expression relative to other hematological malignancies. The cell surface marker CD138 (syndecan-1 (SDC1)) was specifically expressed in MM, and SDC1 was among the 24 genes that were identified. A t test of MM and other hematological malignancies was performed, and the gene with the lowest p value was Gas6. The TAM receptor Mer was overexpressed in 23 of 26 MM cases, and there was a positive correlation between high expression of Gas6 and that of Mer (Fig. 1B). Immunohistochemical analysis indicated that Gas6 protein was expressed in a distribution consistent with that in the CD138-positive cells, which were obtained from the BM specimens of the MM patients (Fig. 1C, a–d). Furthermore, as shown in Fig. 1D, the serum levels of Gas6 protein were markedly increased in 42 symptomatic MM patients compared with 14 healthy volunteers, as determined by a human Gas6 ELISA kit (33.4 ± 14.6 pg/ml in the healthy volunteers and 144.4 ± 92.1 pg/ml in the symptomatic MM patients).
FIGURE 1.
Gas6 was highly expressed in BM and serum of MM patients. A, relative expression ratios are indicated by the color bar below the heat map. Red indicates a higher level of expression, whereas blue indicates a lower level of expression. White indicates unavailable data. The genes are in ascending order based on the p value from the t test of MM versus other hematological malignancies. ALL, acute lymphoid leukemia; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia. B, the TAM receptor Mer and Gas6 expressed in the BM of MM patients. Relative expression ratios are indicated by the color bar below the heat map. Red indicates a higher level of expression, whereas blue indicates a lower level of expression. C, Gas6 expression in BM of MM patients by immunohistochemistry. BM specimens enriched with plasma cells from MM patients were reacted with mouse monoclonal antibody against CD138 or Gas6. Representative photomicrographs are shown (a and c, CD138; b and d, Gas6). D, serum levels of Gas6 in MM patients. Serum levels of Gas6 protein were quantified in healthy volunteers (n = 14) and MM patients (n = 42) by ELISA. Data are expressed as means ± S.D. *, p < 0.05.
Gas6 Evades the Apoptosis and Induces Cell Proliferation in MM Cell Line RPMI-8226
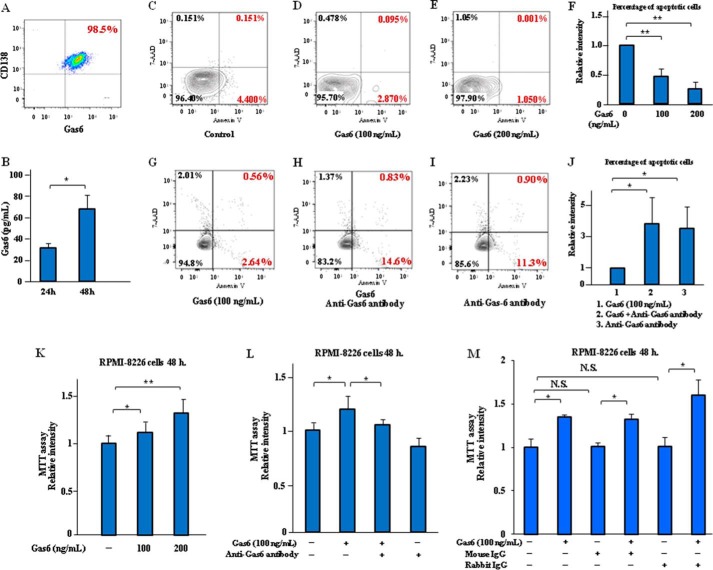
Gas6 was expressed in CD138-positive MM cell line RPMI-8226 (Fig. 2A), and the secretion of soluble Gas6 was significantly elevated in the MM cells after 24–48 h of incubation in the RPMI-8226 cells in the culture supernatants (Fig. 2B). Exogenous Gas6 significantly decreased the number of RPMI-8226 cells in the early and late stages of apoptosis (Annexin V-positive and 7-aminoactinomycin D (7-AAD)-negative) induced by serum deprivation when compared with the control cells (Fig. 2, C–F), and Gas6-neutralizing antibody suppressed anti-apoptosis induced by Gas6 in the RPMI-8226 cells (Fig. 2, G–J). Moreover, exogenous Gas6 significantly prompted the cell proliferation of the MM cells, which was suppressed by Gas6 neutralizing antibody, as determined using MTT cell proliferation assay (Fig. 2, K and L). Mouse IgG and rabbit IgG isotype control antibodies were used as negative controls in the MTT cell proliferation assay. We confirmed that the treatment with these antibodies had no direct effect on Gas6-induced MM cell growth (Fig. 2M). These results indicate that Gas6 may be a critical modifier for cell apoptosis and proliferation of MM cells.
FIGURE 2.
Gas6 promoted anti-apoptosis and cell proliferation of MM cell lines RPMI-8226. A, MM cell lines RPMI-8226 were incubated for 24 h in serum-free X-VIVO medium, and then FCM analysis was performed. Representative data are from three independent experiments are shown. B, RPMI-8226 cells were cultured with serum-free X-VIVO medium for 24–48 h, and Gas6 levels of culture supernatants were quantified by ELISA. Representative data are from four independent experiments. Data are expressed as means ± S.D. (error bars) (n = 8, each group). *, p < 0.05. C, MM cells were incubated in serum-free X-VIVO medium with Gas6 (0–200 ng/ml) for 24 h, and cell apoptosis was analyzed by FCM after staining with Annexin V and 7-AAD. Representative data are from four independent experiments. C, untreated RPMI-8226 cells. D, RPMI-8226 cells treated with 100 ng/ml Gas6. E, RPMI-8226 cells treated with 200 ng/ml Gas6. F, the statistical analysis indicated the effect of exogenous Gas6 on the cell apoptosis of the MM cell line RPMI-8226 cells in a dose-dependent manner. Results are shown as mean ± S.D. of statistical analyses from four separate experiments. **, p < 0.01. G, cell apoptosis was analyzed by FCM after staining with Annexin V and 7-AAD. Representative data are from four independent experiments. RPMI-8226 cells were treated with 100 ng/ml Gas6. H, RPMI-8226 cells treated with 100 ng/ml Gas6 plus 20 μg/ml anti-Gas6 antibody. I, RPMI-8226 cells treated with 100 ng/ml Gas6 plus 10 μg/ml anti-IL-6 antibody. J, the statistical analysis indicated the effect of Gas6 and IL-6 inhibition on cell apoptosis reduced by exogenous Gas6 in RPMI-8226 cells. Results are shown as mean ± S.D. of statistical analyses from four separate experiments. *, p < 0.05. K, the cells were preincubated with or without recombinant Gas6 (100–200 nmol/liter) for 48 h at 37 °C in serum-free X-VIVO medium before the MTT assay as described under “Experimental Procedures.” Data are expressed as means ± S.D. (n = 8, each group). *, p < 0.05; **, p < 0.01. L, RPMI-8226 cells were incubated in serum-free X-VIVO medium in the presence of 100 ng/ml Gas6 with or without neutralizing antibodies to Gas6 (20 μg/ml) for 48 h, followed by an MTT assay. Data are expressed as means ± S.D. (n = 8, each group). *, p < 0.05. M, mouse IgG (10 μg/ml) and rabbit IgG (20 μg/ml) isotype control antibodies are used as negative controls in MTT assay. RPMI-8226 cells were incubated and treated with mouse or rabbit IgG isotype control antibody for 48 h in serum-free X-VIVO medium, followed by MTT assay. Data are expressed as means ± S.D. (n = 8, each group). *, p < 0.05. N.S., not significant.
Roles of Gas6 in Apoptosis Inhibition and Cell Proliferation of MM Cells Induced by BMSC-derived Conditioned Medium (CM)
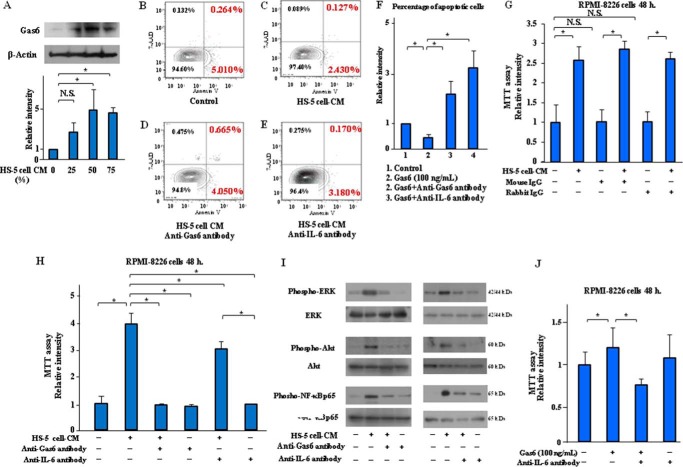
BMSCs, which make up the microenvironment for MM cells, contribute to the anti-apoptosis and cell proliferation of MM cells (19, 20). Signaling stimulation from BMSCs is critical for MM disease progression (21). The Gas6 expression of RPMI-8226 cells was up-regulated by the CM, which was collected from the BMSC cell line HS-5, at 0, 25, 50, and 75% (v/v) of the total medium (Fig. 3A). HS-5 cell-CM decreased the number of RPMI-8226 cells in the early and late stages of apoptosis when compared with the control cells (Fig. 3, B and C), and Gas6-neutralizing antibody inhibited anti-apoptosis induced by HS-5 cell-CM in the RPMI-8226 cells (Fig. 3D), and IL-6-neutralizing antibody also suppressed anti-apoptosis induced by HS-5 cell-CM in the RPMI-8226 cells (Fig. 3E). An MTT cell proliferation assay showed that HS-5 cell-CM induced a significant, ∼4-fold, increase in the cell proliferation of the RPMI-8226 cells, and Gas6-neutralizing antibody markedly decreased the cell proliferation capacity of the MM cells (Fig. 3H, lane 3). We confirmed that the treatment with mouse or rabbit IgG isotype control antibody had no direct effect on MM cell growth induced by HS-5 cell-CM (Fig. 3G). IL-6 is identified as a major growth and anti-apoptotic factor of MM cells through autocrine mechanisms (22, 23). Conversely, BMSCs provide efficient support for MM cell survival by paracrine IL-6 stimulation (24). IL-6-neutralizing antibody suppressed the apoptosis inhibition and cell proliferation of MM cells induced by HS-5 cell-CM (Fig. 3, E and H, lane 5). The mitogen-activated protein kinase (MAPK), originally called ERK, is an extracellular signal-regulated kinase that comprises several key signaling components and phosphorylation events, which play a critical role in cancer cell proliferation and tumorigenesis (25). Nuclear factor-κB (NF-κB) is also a known mediator that promotes cell survival in response to many survival stimuli and is indirectly activated by Akt through direct phosphorylation and activation of IκB kinase (23, 26, 27). HS-5 cell-CM induced ERK, Akt, and NF-κB phosphorylation in RPMI-8226 cells within 45 min after incubation, and the neutralizing antibodies to Gas6 or IL-6 suppressed these signaling pathways (Fig. 3I). Our MTT assay results additionally indicated that IL-6-neutralizing antibody has a similar effect to Gas6-neutralizing antibody in blocking the cell proliferation of MM cells (Fig. 3J). These results suggest that Gas6, as well as IL-6, may play a major role in the pathogenesis of apoptosis inhibition and cell proliferation of MM cells through paracrine mechanisms.
FIGURE 3.
Gas6 and IL-6 inhibition suppressed anti-apoptosis and cell proliferation of MM cells induced by BMSCs-derived CM. The CM from HS-5 cells was collected after a 72-h incubation in serum-free X-VIVO medium. A, RPMI-8226 cells were incubated for 24 h with or without the CM at a level of 25, 50, and 75% (v/v) of the total medium, followed by Western blotting. Results are shown as mean ± S.D. (error bars) of quantitative densitometric analyses from three separate experiments. *, p < 0.05. N.S., not significant. RPMI-8226 cells were incubated for 24 h with or without the CM at a level of 50% (v/v) of the total medium. Cell apoptosis was analyzed by FCM after staining with Annexin V and 7-AAD. Representative data are from three independent experiments. B, untreated RPMI-8226 cells. C, RPMI-8226 cells treated with HS-5 cell-CM. D, RPMI-8226 cells treated with HS-5 cell-CM plus 20 μg/ml anti-Gas6 antibody. E, RPMI-8226 cells treated with HS-5 cell-CM plus 10 μg/ml anti-IL-6 antibody. F, statistical analysis indicated the effect of Gas6 and IL-6 inhibition on cell apoptosis reduced by HS-5 cell-CM in RPMI-8226 cells. Results are shown as mean ± S.D. of statistical analyses from four separate experiments. *, p < 0.05. G, mouse IgG (10 μg/ml) and rabbit IgG (20 μg/ml) isotype control antibodies are used as negative controls in MTT assay. RPMI-8226 cells were incubated in serum-free X-VIVO medium or 50% (v/v) HS-5 cell-CM with or without mouse or rabbit IgG isotype control antibody for 48 h, followed by an MTT assay. Data are expressed as means ± S.D. (n = 8, each group). *, p < 0.05. H, RPMI-8226 cells were incubated in serum-free X-VIVO medium or 50% (v/v) HS-5 cell-CM with or without neutralizing antibodies to Gas6 or IL-6 for 48 h, followed by an MTT assay. Data are expressed as means ± S.D. (n = 8, each group). *, p < 0.05. I, RPMI-8226 cells were incubated with or without 50% (v/v) HS-5 cell-CM with or without neutralizing antibodies to Gas6 or IL-6 for 45 min, followed by Western blotting. Data shown are representative of three independent experiments. J, anti-IL-6 antibody inhibited the cell proliferation of MM cells, indicating an effect of anti-IL-6 antibody on the blocking of MM cell growth similar to that of anti-Gas6 antibody. Data are expressed as mean ± S.D. (n = 4, each group). *, p < 0.05.
Autocrine and Paracrine Actions of Gas6 Mediated via IL-6 on Molecular Interactions between MM Cells and BMSCs in the Pathogenesis of MM
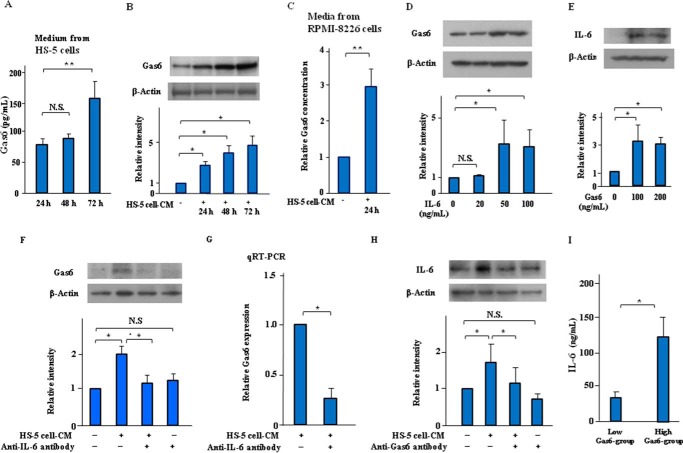
Soluble forms of Gas6 protein were synthesized by the BMSC cell line HS-5 as well as through MM cell line RPMI-8226 (Fig. 4A). Furthermore, Gas6 protein was significantly increased in the RPMI-8226 cells treated with HS-5 cell-CM (Fig. 4B), and HS-5 cell-CM markedly induced a 3-fold increase in the production of soluble Gas6 in culture medium from the RPMI-8226 cells (Fig. 4C). Twenty-four hours of incubation with exogenous IL-6 (50 ng/ml) induced Gas6 up-regulation in the RPMI-8226 cells (Fig. 4D). Exogenous Gas6 (100 ng/ml) significantly increased IL-6 expression in the RPMI-8226 cells (Fig. 4E). We also showed that HS-5 cell-CM induced Gas6 up-regulation in the RPMI-8226 cells (Fig. 4F, lane 2). Interestingly, the Gas6 up-regulation induced by HS-5 cell-CM was suppressed by IL-6-neutralizing antibody (Fig. 4F, lane 3), and quantitative RT-PCR (qRT-PCR) showed that IL-6-neutralizing antibody suppressed increased mRNA levels of Gas6 induced by HS-5 cell-CM in the RPMI-8226 cells (Fig. 4G). Fig. 4H showed that HS-5 cell-CM induced an increase in IL-6 expression, which was suppressed by the Gas6-neutralizing antibody. In additioņ ELISA showed that the serum levels of IL-6 protein were significantly increased in the high-Gas6 group (≥100 pg/ml) compared with the low-Gas6 group (<100 pg/ml) of symptomatic MM patients (Fig. 4I). Taken together, these results suggest that Gas6 may have both autocrine and paracrine functions in the pathogenesis of MM by regulating myeloma cell growth factors, such as IL-6, triggered by interactions between MM cells and BMSCs.
FIGURE 4.
Autocrine and paracrine actions of Gas6 in cooperation with IL-6 on molecular cross-talk between MM cells and BMSCs in the pathogenesis of MM. A, HS-5 cells were indicated in serum-free X-VIVO medium for up to 72 h. Gas6 protein in culture supernatants from HS-5 cells were quantified by Gas6 ELISA. Data are expressed as means ± S.D. (error bars) (n = 8, each group). **, p < 0.01. N.S., not significant. B, RPMI-8226 cells were incubated with or without 50% (v/v) HS-5 cell-CM in serum-free X-VIVO medium for up to 72 h, followed by Western blotting. Immunoblots are from an experiment representative of three similar experiments. Bars, means ± S.D. of quantitative densitometric analyses. *, p < 0.05. C, RPMI-8226 cells were incubated with or without 50% (v/v) HS-5 cell-CM for 24 h. Soluble Gas6 concentration in culture supernatants from RPMI-8226 cells were quantified by Gas6 ELISA. Data are expressed as means ± S.D. (n = 8, each group). **, p < 0.01. D, RPMI-8226 cells were incubated in serum-free X-VIVO medium with or without IL-6 (0–100 ng/ml) for 24 h, followed by Western blotting. Data shown are representative of three independent experiments. Bars, mean ± S.D. of quantitative densitometric analyses. *, p < 0.05. E, RPMI-8226 cells were incubated in serum-free X-VIVO medium with or without Gas6 (0–200 ng/ml) for 24 h, followed by Western blotting. Results are shown as mean ± S.D. of quantitative densitometric analyses from three separate experiments. *, p < 0.05. F, RPMI-8226 cells were incubated in serum-free X-VIVO medium or 50% (v/v) HS-5 cell-CM with or without IL-6-neutralizing antibody (10 μg/ml) for 24 h. Immunoblots are from an experiment representative of three similar experiments. Bars, means ± S.D. of quantitative densitometric analyses. *, p < 0.05. G, RPMI-8226 cells were incubated in the presence of HS-5 cell-CM with or without IL-6-neutralizing antibody (10 μg/ml) for 24 h, followed by qRT-PCR. Representative data are from four independent experiments. Data are expressed as means ± S.D. *, p < 0.05. H, RPMI-8226 cells were incubated in serum-free X-VIVO medium or 50% (v/v) HS-5 cell-CM with or without Gas6-neutralizing antibody (20 μg/ml) for 24 h, followed by Western blotting. Immunoblots are from an experiment representative of three similar experiments. Bars, means ± S.D. of quantitative densitometric analyses. *, p < 0.05. I, serum levels of IL-6 protein in symptomatic MM patients (n = 14) were quantified in the high-Gas6 group (≥100 pg/ml) compared with the low-Gas6 group (<100 pg/ml), as determined by a human IL-6 ELISA kit. Data are expressed as means ± S.D. *, p < 0.05.
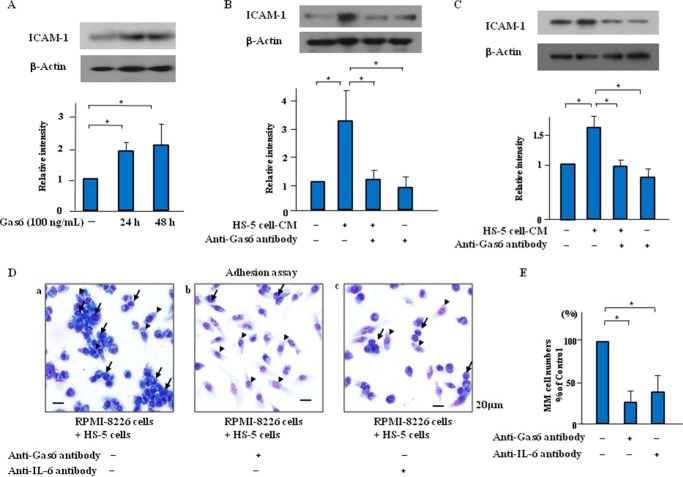
Gas6-neutralizing Antibody Suppressed ICAM-1 Up-regulation Induced by HS-5 Cell-CM in MM Cells in Vitro
ICAM-1 enhanced the adhesion of MM cells to BMSCs and subsequent MM disease progression (28). In the present study, exogenous Gas6 significantly induced ICAM-1 up-regulation in the RPMI-8226 cells in a time-dependent manner (Fig. 5A). Treatment with HS-5 cell-CM induced the up-regulation of ICAM-1, which was reversed by neutralizing antibodies to Gas6 or IL-6 in the RPMI-8226 cells (Fig. 5, B and C). Our data indicate that Gas6 stimulation may increase ICAM-1 synthesis and subsequently accelerate the proliferation of the MM cells. In co-culture of RPMI-8226 cells and HS-5 cells, the adherence of the RPMI-8226 cells to the HS-5 cells was promoted and subsequently induced the cell proliferation of the RPMI-8226 cells (Fig. 5, D and E). The neutralizing antibodies to Gas6 or IL-6 markedly reduced the adherence of the RPMI-8226 cells to the HS-5 cells and subsequent cell proliferation of the RPMI-8226 cells (Fig. 5, D and E).
FIGURE 5.
Gas6 and IL-6 inhibition suppressed ICAM-1 up-regulation and cell survival induced by HS-5 cell-CM in MM cells. A, RPMI-8226 cells were incubated in serum-free X-VIVO medium with or without Gas6 (100 ng/ml) for up to 48 h, followed by Western blotting. Results are the means ± S.D. (error bars) of quantitative densitometric analyses from three separate experiments. *, p < 0.05. B and C, RPMI-8226 cells were incubated in serum-free X-VIVO medium or 50% (v/v) HS-5 cell-CM with or without the neutralization antibodies to Gas6 (20 μg/ml) or IL-6 (10 μg/ml) for 24 h, followed by Western blotting. Representative immunoblots are from three similar experiments are shown. Bars, means ± S.D. of quantitative densitometric analyses. *, p < 0.05. D, direct cell-cell contact between RPMI-8226 cells and HS-5 cells. RPMI-8226 cells and HS-5 cells co-cultured with or without the neutralization antibodies to Gas6 (20 μg/ml) or IL-6 (10 μg/ml) for 24 h, followed by May-Grünwald-Giemsa staining. D, photomicrographs are from an experiment representative of three independent experiments (a, control; b, anti-Gas6 antibody (20 μg/ml for 24 h); c, anti-IL-6 antibody (10 μg/ml for 24 h)). Arrow, RPMI-8226 cells; arrowhead, HS-5 cells. E, the number of adhered cells was quantified by direct visualization of five. Data are expressed as means ± S.D. **, p < 0.01.
Critical Role of Gas6/Mer Axis in the Pathogenesis of MM
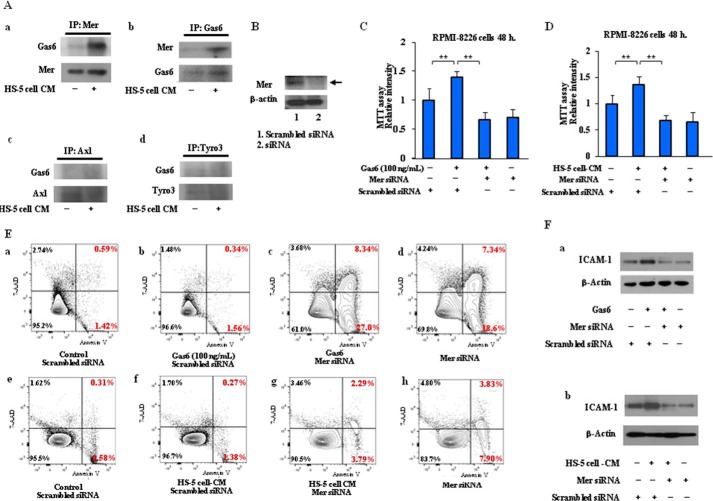
To identify which of the receptor tyrosine kinases Mer, Axl, and Tyro3 (TAM receptors) contribute to Gas6-mediated signaling, immunoprecipitation studies were performed with RPMI-8226 cells. As shown in Fig. 6A (a), Gas6 was markedly expressed in the immunoprecipitated proteins with anti-Mer antibody in RPMI-8226 cells treated with HS-5 cell-CM, whereas little to no Gas6 expression was detectable in the immunoprecipitated proteins with anti-Axl or anti-Tyro3 antibody in RPMI-8226 cells (Fig. 6A, c and d). The data indicate that HS-5 cell-CM stimulates the binding of Gas6 to Mer receptor in RPMI-8226 cells. To further investigate the association between Gas6 and Mer, we transfected RPMI-8226 cells with Mer siRNA and then added exogenous Gas6 or HS-5 cell-CM to RPMI-8226 cells and found that siRNA-mediated knockdown of Mer significantly reduced Mer protein (Fig. 6B). The MTT assay showed that 48 h of treatment with Gas6 or HS-5 cell-CM induced cell proliferation, which was blocked by the silencing of Mer with siRNA (Fig. 6, C and D). Also, the anti-apoptosis induced by exogenous Gas6 or HS-5 cell-CM was suppressed by Mer siRNA in the early and late stages of apoptosis (Fig. 6E). In addition, we showed that ICAM-1 up-regulation, which was induced by Gas6 or HS-5 cell-CM, was inhibited by Mer siRNA (Fig. 6F). These results indicate that Gas6/Mer signaling axis is a key modifier in the pathogenesis of MM through autocrine and paracrine mechanisms.
FIGURE 6.
Critical role of the TAM family receptor Mer in Gas6-related signaling pathways in the pathogenesis of MM. A, the lysates were immunoprecipitated (IP) with anti-Mer monoclonal antibody, and immunoprecipitates were analyzed by Western blotting with anti-Gas6 antibody. Gas6 expression was detected in the subsequent immunoprecipitation with anti-Mer antibody in MM cells treated with HS-5 cell-CM, whereas very little or no Gas6 expression was detectable in the immunoprecipitated proteins with anti-Axl or anti-Tyro3 antibody in MM cells. B, RPMI-8226 cells were transfected with siRNA to Mer. siRNA-mediated knockdown of Mer reduced the protein levels of Mer as compared with the scrambled negative control. C and D, MM cells were incubated in serum-free X-VIVO medium for 48 h at 37 °C in the presence of Gas6 (100 ng/ml) or 50% (v/v) HS-5 cell-CM with or without the silencing of Mer with siRNA, followed by an MTT assay. Data are expressed as means ± S.D. (error bars) (n = 8, each group). *, p < 0.05. E, MM cells were incubated in serum-free X-VIVO medium in the presence of Gas6 (100 ng/ml) or 50% (v/v) HS-5 cell-CM for 24 h with or without the silencing of Mer with siRNA, and cell apoptosis was analyzed by FCM after staining with Annexin V and 7-AAD. Representative data are from three independent experiments. Data shown are representative of three independent experiments. F, RPMI-8226 cells were incubated in serum-free X-VIVO medium in the presence of Gas6 (100 ng/ml) or 50% (v/v) HS-5 cell-CM for 24 h with or without the silencing of Mer with siRNA, followed by Western blotting. Representative immunoblots are from three similar experiments are shown.
Effects of Mer siRNA on ERK, Akt, and NF-κB phosphorylation in MM cells stimulated by Gas6 or HS-5 cell-CM
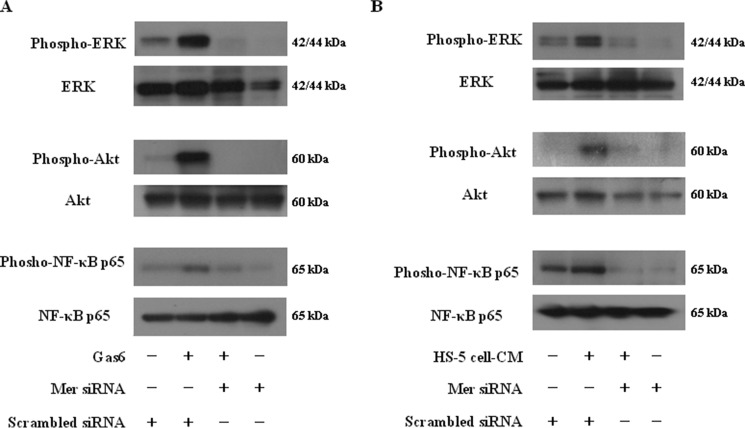
We further examined whether the TAM family receptor Mer is critical for Gas6-activated downstream signaling pathways in MM cells. siRNA-mediated suppression of Mer inhibited the increase in ERK, Akt, and NF-κB phosphorylation in RPMI-8226 cells stimulated by Gas6 or HS-5 cell-CM within 45 min after incubation (Fig. 7, A and B). The data indicate the significance of Mer in the molecular pathogenesis of MM.
FIGURE 7.
Effects of Mer siRNA on ERK, Akt, and NF-κB phosphorylation in MM cells stimulated by Gas6 or HS-5 cell-CM. A and B, RPMI-8226 cells were incubated in the presence of Gas6 (100 ng/ml) or 50% (v/v) HS-5 cell-CM for 45 min with or without the silencing of Mer with siRNA, followed by Western blotting with the antibodies to phospho-ERK, phospho-Akt, and phospho-NF-κB.
Discussion
In the present study, we demonstrated that the molecular cross-talk/interaction between MM cells and BMSCs stimulated Gas6 production, which stimulated the development, maintenance, and progression of MM via autocrine and paracrine mechanisms.
Gene expression signatures have demonstrated that expression profiling can be used to identify patients with a high risk of disease, to guide therapeutic interventions, and to stratify patients into clinically relevant molecular subgroups in many types of cancers, including newly diagnosed MM (29, 30). To identify genes that are specifically expressed at high levels in MM, we screened molecules that were differentially expressed in the BM cells of MM patients using microarray analysis. Our heat map revealed significant up-regulation of Gas6 in MM compared with other hematological malignancies (Fig. 1A). Gas6 has been reported to function as a signaling molecule to regulate tumor cell proliferation, anti-apoptosis, and invasion as well as a negative coregulator in blood coagulation (31). Among hematological malignancy cell lines, Gas6 mRNA expression was only detected in 4 of 22 lymphoid cell lines and in 4 of 17 myeloid lines; however, plasma cell lineages revealed that Gas6 mRNA was expressed in all four investigated cell lines (32). This result is almost identical to our finding of a significant difference between Gas6 expression levels in BM from patients with MM and those with other types of hematological malignancy in the DNA microarray analysis (Fig. 1A). The involvement of Gas6 in myeloma cell proliferation has recently been reported, in which Mer was expressed, whereas Axl and Tyro3 were not or were expressed by a low proportion in MM cells (3). In our DNA microarray analysis of myeloma patients, Mer was overexpressed in 23 of 26 myeloma patients, suggesting that Mer potentially plays important roles in the Gas6-related signaling pathways of MM cells (Fig. 1B). In the present study, we obtained the following findings: 1) in the BM of the myeloma patients, Gas6 protein was expressed in a distribution consistent with that in the CD138-positive cells, which indicate MM cells (Fig. 1C); 2) Gas6 plasma levels were significantly higher in the MM patients than in the healthy subjects (Fig. 1D); 3) endogenous Gas6 was expressed in the CD138-positive MM cell lines (Fig. 2A); 4) Gas6 was detected in the supernatants of in vitro cultured MM cells (Fig. 2B); 5) the addition of exogenous Gas6 on cultured MM cells suppressed apoptosis induced by serum deprivation (Fig. 2, C–F), and exogenous Gas6 stimulated cell proliferation (Fig. 2K). Moreover, Gas6-neutralizing antibody suppressed the anti-apoptosis (Fig. 2, G–J) and cell proliferation of the RPMI-8226 cells (Fig. 2L), suggesting the presence of an autocrine loop of Gas6 in the cell survival of MM cells. Our findings support a recent report demonstrating that autocrine action of Gas6 mediated via its receptor Mer promotes proliferation and survival in MM cells (3).
The molecular cross-talk/interaction between MM cells and the surrounding BM microenvironment has been shown to contribute to the pathogenesis of MM (19–21, 33). BMSCs isolated from MM patients are phenotypically and functionally altered compared with those from healthy donors (34, 35). The anti-apoptotic and proliferative effects are dependent on paracrine functions of BMSC-derived cytokines, including IL-6 (24). IL-6 secretion by both MM cells and BMSCs mediates autocrine and paracrine growth of MM cells and inhibit tumor cell apoptosis (24, 36). We showed that 50% (v/v) HS-5 cell-CM significantly up-regulated the Gas6 expression of MM cells (Fig. 3A). The treatment with 50% (v/v) HS-5 cell-CM partially suppressed apoptosis induced by serum deprivation and stimulated proliferation (Fig. 3, C and G (lane 2)). These HS-5 cell-CM effects were inhibited by neutralizing antibodies to Gas6 or IL-6 (Fig. 3, D, E, and G (lanes 5 and 6)), indicating that MM cells receive anti-apoptotic and proliferative signals from autocrine and paracrine stimulation of Gas6, which served functions similar to those of IL-6.
The Ras/MEK/ERK pathway provides a stimulus for mitochondrial dysfunction and apoptosis as well as proliferation in MM cells, and the ERK signaling pathway contributes to the mechanism(s) of relapsing and refractory MM (37–39). On the other hand, c-Jun N-terminal kinase and p38 MAPK have been reported to induce G2/M progression delay and cell apoptosis in MM cells (40). The canonical NF-κB pathway is negatively regulated by inhibitory IκB proteins (IκBs, inhibitors of NF-κB), which retain p50/p65-containing heterodimers (41). In the non-canonical NF-κB pathway, a mitogen-activated protein kinase kinase kinase 14/NF-κB-inducing kinase (NIK) activates IκB kinase-α (IKKα), which induces the nuclear factor κ-B subunit 2/p100 phosphorylation and processing (42). Inhibition of both the canonical and non-canonical pathways is required to efficiently block total NF-κB in the pathology of MM (43). Because myeloma as well as many other B-cell neoplasms use NF-κB to achieve survival, proliferation, and resistance to anticancer drugs, NF-κB pathways appear to be an attractive therapeutic approach in MM tumors (23, 26, 27, 44, 45). The first therapeutic proteasome inhibitor, bortezomib, exhibited inhibitory effects on the activity of two major NF-κB complexes, p50/p65 (RelA) and p52/RelB dimers, in the pathogenesis of MM (46). Bruton's tyrosine kinase-driven NF-κB p65 activity plays a critical role in bortezomib-resistant MM cells (47). BMSCs from MM patients uniquely induce bortezomib-resistant NF-κB activity in RPMI-8226 cells (48). Phosphatidylinositol 3-kinase/Akt/NF-κB signaling is involved in the pathogenesis of MM (27, 37). Our results showed that HS-5 cell-CM induced the phosphorylation of ERK, Akt, and NF-κB, which were inhibited by neutralizing antibodies to Gas6 or IL-6 in the MM cells within 45 min of incubation (Fig. 3H), indicating that paracrine stimulation of BMSCs promoted survival of the MM cells via phosphorylation of ERK, Akt, and NF-κB.
The quantitative determination by ELISA showed that soluble Gas6 protein levels were markedly increased in the CM from human BMSC line HS-5 after a 72-h incubation (Fig. 4A). Additionally, we found that HS-5 cell-CM significantly induced Gas6 up-regulation in the RPMI-8226 cells (Fig. 4B), and HS-5 cell-CM markedly increased soluble Gas6 in culture supernatants of the MM cells after a 24-h incubation (Fig. 4C). Our data suggest that BMSCs may not only secrete Gas6 but also induce elevated Gas6 synthesis in MM cells. Meanwhile, IL-6 (50 ng/ml) and 50% (v/v) HS-5 cell-CM induced Gas6 up-regulation in the RPMI-8226 cells after a 24-h incubation (Fig. 4, D, F (lane 2), and H (lane 2)), and the Gas6 up-regulation induced by HS-5 cell-CM was suppressed by IL-6-neutralizing antibody (Fig. 4, F (lane 3) and G (lane 2)). Crucially, HS-5 cell-CM induced IL-6 up-regulation in the RPMI-8226 cells, which was suppressed by Gas6-neutralizing antibody (Fig. 4H, lane 3). In addition, Fig. 4I shows the serum levels of IL-6 protein in symptomatic MM patients in the high-Gas6 group (≥100 pg/ml) and in the low-Gas6 group (<100 pg/ml). Our data indicate that the effects of Gas6 on the growth and survival of MM cells may be mediated not only via direct stimulation, but also via elevation of IL-6 expression in MM cells. Based on the data presented in the current study, we suggest that Gas6 may be a major modulator in the pathogenesis of the proliferation and apoptosis inhibition of MM cells through autocrine and paracrine interactions between MM cells and BMSCs in analogy with IL-6.
ICAM-1/MUC1 is important in the adhesion between MM cells and BMSCs, and ICAM-1 is involved in cell-adhesive events that trigger multiple cell-signaling pathways, promoting MM cell proliferation, migration, and resistance to apoptosis (28, 49). ICAM-1 overexpression relates to MM progression and chemotherapeutic resistance, and its levels are associated with international staging system scores. In addition, BI-505, an anti-ICAM-1 human monoclonal antibody, possesses anti-myeloma activity and is already being used clinically (50). Fig. 5 (B and C) shows that ICAM-1 up-regulation induced by HS-5 cell-CM was reversed by neutralizing antibodies to Gas6 or IL-6 in RPMI-8226 cells, indicating that paracrine Gas6 stimulation enhanced adhesion of the MM cells to the BMSCs by increasing ICAM-1 synthesis and MM progression. Moreover, in the co-culture system of the MM cells and BMSCs, the adherence of the MM cells to BMSCs and subsequent cell proliferation of the MM cells were promoted, and neutralizing antibodies to Gas6 or IL-6 suppressed the adherence and cell proliferation (Fig. 5, D and E). These results indicate that Gas6 or IL-6 inhibition suppressed the adherence of the MM cells to the BMSCs and subsequent cell proliferation of the MM cells. Moreover, siRNA-mediated suppression of Mer tyrosine kinase receptor inhibited the 1) cell proliferation and 2) apoptosis inhibition of MM cells induced by Gas6 or HS-5 cell-CM (Fig. 6, C–E), and ICAM-1 up-regulation induced by Gas6 or HS-5 cell-CM was reversed by Mer siRNA in MM cells (Fig. 6F).
The prosurvival factors, such as ERK, Akt, and NF-κB, are involved in the regulation of proliferation as well as drug resistance of MM cells (39, 40, 45–47). We showed that Mer siRNA inhibited the increase in ERK, Akt, or NF-κB phosphorylation in MM cells stimulated by Gas6 or HS-5 cell-CM (Fig. 7, A and B). These results indicate that Gas6/Mer-mediated activation of ERK, Akt, and NF-κB plays a critical role in the pathogenesis of MM.
It has been reported that individuals with elevated Gas6 have an increased risk of venous thromboembolism compared with those with lower levels of Gas6, indicating that elevated Gas6 may be a causal factor in developing venous thromboembolism associated with MM (51). In addition, Katagiri et al. (52) reported that Gas6/Tyro3 signaling stimulates osteoclastic bone resorption in mouse osteoclasts. These studies suggest that elevated expression levels of Gas6 may be critical not just for MM progression, but for complications of MM, although further investigation is needed.
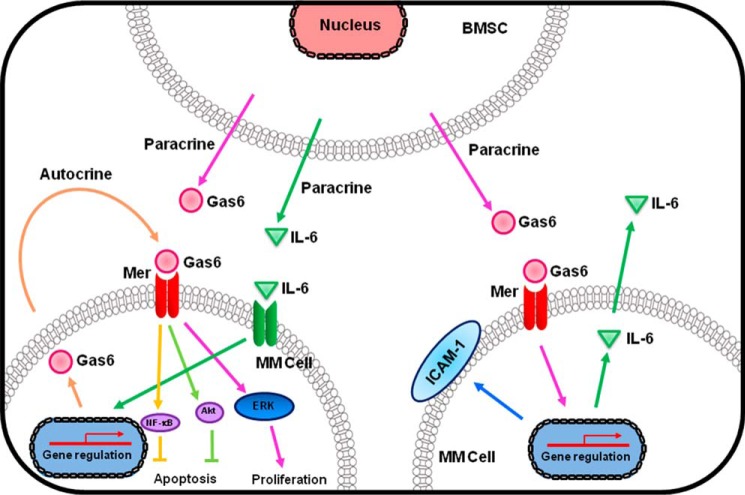
The present study offers new insights into the autocrine and paracrine actions of Gas6 in concert with IL-6 in both MM cells and BMSCs on apoptosis inhibition and cell proliferation of MM cells (Fig. 8). Our findings also suggest that Gas6/Mer downstream signaling pathways in concert with IL-6 may be crucial for the progression of MM, and therefore they are attractive therapeutic targets in the treatment of MM.
FIGURE 8.
Schematic diagram describing the autocrine and paracrine mechanisms of Gas6 in cooperation with IL-6 on molecular cross-talk/interaction between MM cells and BMSCs in the pathogenesis of MM. The autocrine and paracrine actions of Gas6 in concert with IL-6 in both MM cells and BMSCs enhance proliferation and inhibition of apoptosis via phosphorylation of ERK, Akt, and NF-κB in MM cells. The effects of Gas6 are mediated via the Mer tyrosine kinase receptor in MM cells. In addition, paracrine stimulation of Gas6 enhances adhesion of the MM cells to the BMSCs by increasing synthesis of ICAM-1 and IL-6 and leads to subsequent MM disease progression.
Experimental Procedures
Patient Samples
This study was approved by the Fukushima Medical University institutional review board. BM and/or peripheral blood samples were obtained from MM patients with informed consent. Samples from 13 healthy individuals were used as controls.
Cell Culture and Reagents
Human MM cell lines RPMI-8226 (5 × 105 cells/ml) were cultured in RPMI-1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 5% penicillin-streptomycin (Gibco) or serum-free X-VIVO medium (Lonza, Walkersville, MD) at 37 °C with 5% CO2. Human BMSC line HS-5 (5 × 105 cells/ml) was incubated in RPMI-1640 medium supplemented with 10% fetal bovine serum and 5% penicillin-streptomycin at 37 °C with 5% CO2, and then conditioned medium from the confluent HS-5 cells were collected after 72 h of culture in serum-free X-VIVO medium. Recombinant human Gas6 and IL-6 were purchased from R&D Systems, Inc. (Minneapolis, MN). We used the neutralization antibodies to Gas6 (Bioworld Technology) and IL-6 (R&D Systems). Mouse IgG1 isotype control antibody and rabbit IgG isotype control antibody (SouthernBiotech, Homewood, AL) were used.
DNA Microarray
BM or blood that had been mixed with an equal volume of water was mixed with 3 times the volume of ISOGEN-LS reagent (NIPPON GENE, Tokyo, Japan). Total RNA was prepared from the lysate according to the manufacturer's instructions. Poly(A)+ RNA was purified using the Poly(A) Purist Kit (Ambion, Austin, TX). To reduce the bias in cell type-specific expression, human common reference RNA was prepared by mixing equal amounts of poly(A)+ RNA extracted from 22 cell lines (53). Synthetic polynucleotides (80-mers) representing 30,913 species of human transcripts (MicroDiagnostic, Tokyo, Japan) were arrayed by using a custom arrayer. With 2 μg of RNA serving as a template, the cDNA from the sample RNA was labeled with cyanine 5-dUTP (PerkinElmer Life Sciences), and that from the reference RNA was labeled with cyanine 3-dUTP (PerkinElmer Life Sciences). The labeled cDNA was then synthesized using SuperScript II reverse transcriptase (Life Technologies, Inc.). Hybridization was performed using a labeling and hybridization kit (MicroDiagnostic). Fluorescence was visualized using a GenePix 4000B scanner, and fluorescence intensity was expressed numerically as the ratio of cyanine 5 fluorescence to cyanine 3 fluorescence. Each ratio was adjusted by multiplying the normalization factor, which is calculated by GenePix Pro version 3.0 software (Axon Instruments Inc.). This ratio was converted to a log2 value and normalized so that the mean would be 0 and the S.D. would be 1. Spots where the fluorescence intensity was less than the limits of detection were assigned a log ratio of 0 and included in calculations. Multiple group comparisons of MM and other hematological malignancies were performed using the Tukey-Kramer method. If fluorescence was not detected in ≥20% of the samples, then that gene was excluded from the comparison analysis.
Immunostaining
BM specimens enriched with plasma cells from MM patients were fixed for 15 min with 4% paraformaldehyde (Merck, Frankfurt, Germany) in phosphate buffer (pH 7.2). After rinsing with PBS, the cells were embedded in paraffin for immunohistochemistry. Sections were prepared, deparaffinized, and autoclaved at 121 °C for 10 min in 10 mm Tris-HCl buffer (pH 9.0) as an antigen retrieval procedure. The cells were then blocked and incubated with goat polyclonal antibody against Gas6 (sc-1935, Santa Cruz Biotechnology, Inc., Dallas, TX) or mouse monoclonal antibody against CD138 (clone MI-15, Dako, Glostrup, Denmark) at 4 °C for 2 days, followed by detection using appropriate streptavidin-biotinylated horseradish peroxidase system (Vectastain Elite ABC kit, Vector Laboratories, Inc., Burlingame, CA). They were visualized with 3,3′-diaminobenzidine (Dojindo Laboratories, Kumamoto, Japan) and hydrogen peroxide and observed with a light microscope (BX51, Olympus, Tokyo, Japan).
Flow Cytometry
To detect cytoplasmic Gas6, the cells were permeabilized with a saponin-based reagent (BD PharMingen) according to the manufacturer's instructions. The cells were treated with anti-Gas6 antibody (Santa Cruz Biotechnology), conjugated to anti-FITC secondary antibody (Santa Cruz Biotechnology), and then stained with anti-CD138-PE antibody (Santa Cruz Biotechnology) using flow cytometry (BD FACSCantoTM II flow cytometer, BD Biosciences). To detect apoptosis, the cells were stained with FITC-conjugated Annexin V and 7-AAD according to the manufacturer's instructions (eBioscience) and analyzed by flow cytometry (FCM).
ELISA
The levels of Gas6 and IL-6 protein were assessed in human plasma and culture supernatant using a commercially available Gas6 ELISA kit (Assay Biotechnology) and IL-6 ELISA kit (Diaclone SAS, Besancon Cedex, France), respectively, in accordance with the manufacturer's instructions. Briefly, 96-well plates were coated with anti-Gas6 antibody, and the antigen was detected by a secondary biotinylated detection antibody. When the substrate 3,3′,5,5′-tetramethylbenzidine was added, the reaction catalyzed by peroxidase yielded a blue color that was representative of the antigen concentration. The reaction was stopped with 2 n sulfuric acid, and the absorbance at 450 nm was read, with a reference wavelength set at 570 nm, using a microplate reader (Molecular Devices).
Cell Proliferation Assay
To examine the effect of recombinant Gas6 on the proliferation of MM cell lines, we performed a MTT cell proliferation assay (Promega Biosciences, Inc.) according to the manufacturer's instructions. The optical density was measured using a spectrophotometer (Molecular Devices), and the -fold increase in the optical density compared with that of the control was calculated.
Western Blotting
Western blotting was performed as described previously (54). The signals from immunoreactive bands were visualized by an ECL System (GE Healthcare). Rabbit polyclonal antibodies to Gas6 (Santa Cruz Biotechnology) were diluted 1:250 in PBS with 5% BSA. ICAM-1 antibody (Santa Cruz Biotechnology) was diluted 1:250 in PBS with 5% BSA. Anti-β-actin antibody (1:1000; Santa Cruz Biotechnology) was used as a loading control. ERK and Akt activation was determined by Western blotting using an anti-phospho-ERK antibody (Ser-536, dilution 1:250, Cell Signaling Technology, Inc., Beverly, MA) and an anti-phospho-Akt antibody (dilution 1:250, Cell Signaling Technology). The anti-phospho-Akt antibody detects endogenous levels of Akt1 only when phosphorylated at Ser-473 and also recognizes Akt2 and Akt3. Anti-Akt antibody (1:250; Cell Signaling Technology) and anti-ERK antibody (1:250; Cell Signaling Technology) were used as a loading control. We used an anti-phospho-NF-κB p65 antibody (Ser-536, dilution 1:250; Cell Signaling Technology), and anti-NF-κB p65 antibody (dilution 1:250; Cell Signaling Technology) was used as a loading control.
Immunoprecipitation
Human MM cell line RPMI-8226 was solubilized in a radioimmune precipitation assay buffer (Sigma-Aldrich), and immunoprecipitation was performed as described previously (55). Briefly, the lysates were reacted with monoclonal antibodies against Mer, Axl, or Tyro3 (Santa Cruz Biotechnology) at a concentration of 1.0 mg/ml. The immunoprecipitated proteins were resolved by SDS-PAGE, followed by Western blotting. We used the primary antibody against Gas6 (dilution 1:250; Santa Cruz Biotechnology), which recognizes the catalytic domain of Gas6 and anti-rabbit IgG, TrueBlotTM (eBioscience, Inc., San Diego, CA).
siRNA
siRNA was transduced in RPMI-8226 cells by electroporation using the Cell Line Nucleofector Kit V (VCA-1003; Lonza) with the Amaxa Nucleofector Device (Lonza). Mer siRNA and control non-silencing siRNA were obtained from Santa Cruz Biotechnology. Briefly, the cells were transfected with 5 μg of siRNA to Mer or control non-silencing siRNA along with 100 μl of transfection solution. The cells were electroporated by the Amaxa Nucleofector device using the manufacturer's recommended program (S-005). At 24 h after transfection, the culture medium was changed.
qRT-PCR of Gas6
qRT-PCR was performed as described previously (56). Total RNA was extracted from MM cell lines using the RNeasy minikit (Qiagen, Hilden, Germany). After treatment with DNase I (Life Technologies), reverse transcription (RT) was performed using a High Capacity cDNA Reverse Transcription Kit (Life Technologies). qRT-PCR was performed with TaqMan Gene Expression Master Mix (Life Technologies) and TaqMan gene expression assays for Gas6 (assay ID: Hs01090305) and HPRT1 (assay ID: Hs99999909) using the Thermal Cycler Dice Real Time System (TP800, Takara, Shiga, Japan). Expression of HPRT1 RNA was used as an internal control.
Co-culture Adhesion Assay
Adherence of MM cell line RPMI-8226 to human BMSC cell line HS-5 monolayer was assessed. We cultured the HS-5 cells on chamber slides for 48 h and added RPMI-8226 cells (2.0 × 105 cells/well) to the HS-5 cells' monolayer for 48 h at 37 °C. Non-adherent MM cells were then removed by three washes with PBS, and May-Grünwald-Giemsa staining was used for morphological inspection. The remaining cells were photographed under a microscope (BX-53–33NC, Olympus Corp., Tokyo, Japan). The number of adherent cells was quantified by direct visualization of five.
Densitometric Analysis
The optical densities of the individual bands in the Western blots were analyzed using the National Institutes of Health IMAGE program (Bethesda, MD). The area of each analyzed band was kept constant for each analyzed blot. Background density was subtracted from the densitometric data obtained for each band.
Statistical Analysis
Statistical analyses were performed using analysis of variance with Scheffé's post hoc test if appropriate. A value of p < 0.05 was considered statistically significant. Data are expressed as means ± S.D.
Author Contributions
M. F. and H. O. designed the experiments, analyzed the data, and wrote the paper. K. I., K. U., A. S.-N., E. I., J. I., Y. Y., R. H., and S. Watanabe performed the experiments. S. Waguri, K. O., T. I., and Y. T. contributed to the design of research.
Acknowledgments
We acknowledge the outstanding technical assistance of Minae Takasaki, Ayumi Haneda, and Chisato Kubo. We also thank Takako Asanuma for histological assistance.
This work was supported by a Fukushima Medical University Research Project and Grants-in-Aid for Scientific Research 2479080 and 16K19583 from the Japan Society for the Promotion of Science. The authors declare that they have no conflicts of interest with the contents of this article.
- MM
- multiple myeloma
- BM
- bone marrow
- BMSC
- bone marrow stromal cell
- NF-κB
- nuclear factor-κB
- ICAM-1
- intercellular cell adhesion molecule-1
- 7-AAD
- 7-aminoactinomycin D
- CM
- conditioned medium
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- qRT-PCR
- quantitative RT-PCR
- FCM
- flow cytometry.
References
- 1. Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., and Tanaka H. (1988) Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 332, 83–85 [DOI] [PubMed] [Google Scholar]
- 2. Hideshima T., Chauhan D., Podar K., Schlossman R. L., Richardson P., and Anderson K. C. (2001) Novel therapies targeting the myeloma cell and its bone marrow microenvironment. Semin. Oncol. 28, 607–612 [DOI] [PubMed] [Google Scholar]
- 3. Waizenegger J. S., Ben-Batalla I., Weinhold N., Meissner T., Wroblewski M., Janning M., Riecken K., Binder M., Atanackovic D., Taipaleenmaeki H., Schewe D., Sawall S., Gensch V., Cubas-Cordova M., Seckinger A., et al. (2015) Role of growth arrest-specific gene 6-mer axis in multiple myeloma. Leukemia 29, 696–704 [DOI] [PubMed] [Google Scholar]
- 4. Schneider C., King R. M., and Philipson L. (1988) Genes specifically expressed at growth arrest of mammalian cells. Cell 54, 787–793 [DOI] [PubMed] [Google Scholar]
- 5. Manfioletti G., Brancolini C., Avanzi G., and Schneider C. (1993) The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol. Cell Biol. 13, 4976–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Meer J. H., van der Poll T., van 't Veer C. (2014) TAM receptors, Gas6, and protein S: roles in inflammation and hemostasis. Blood 123, 2460–2469 [DOI] [PubMed] [Google Scholar]
- 7. Avanzi G. C., Gallicchio M., Cavalloni G., Gammaitoni L., Leone F., Rosina A., Boldorini R., Monga G., Pegoraro L., Varnum B., and Aglietta M. (1997) GAS6, the ligand of Axl and Rse receptors, is expressed in hematopoietic tissue but lacks mitogenic activity. Exp. Hematol. 25, 1219–1226 [PubMed] [Google Scholar]
- 8. Neubauer A., Fiebeler A., Graham D. K., O'Bryan J. P., Schmidt C. A., Barckow P., Serke S., Siegert W., Snodgrass H. R., and Huhn D. (1994) Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood 84, 1931–1941 [PubMed] [Google Scholar]
- 9. Mudduluru G., Ceppi P., Kumarswamy R., Scagliotti G. V., Papotti M., and Allgayer H. (2011) Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene 30, 2888–2899 [DOI] [PubMed] [Google Scholar]
- 10. Jiang T., Liu G., Wang L., and Liu H. (2015) Elevated serum Gas6 is a novel prognostic biomarker in patients with oral squamous cell carcinoma. PLoS One 10, e0133940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sainaghi P. P., Castello L., Bergamasco L., Galletti M., Bellosta P., and Avanzi G. C. (2005) Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J. Cell Physiol. 204, 36–44 [DOI] [PubMed] [Google Scholar]
- 12. Hutterer M., Knyazev P., Abate A., Reschke M., Maier H., Stefanova N., Knyazeva T., Barbieri V., Reindl M., Muigg A., Kostron H., Stockhammer G., and Ullrich A. (2008) Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin. Cancer Res. 14, 130–138 [DOI] [PubMed] [Google Scholar]
- 13. Mc Cormack O., Chung W. Y., Fitzpatrick P., Cooke F., Flynn B., Harrison M., Fox E., Gallagher E., Goldrick A. M., Dervan P. A., Mc Cann A., and Kerin M. J. (2008) Growth arrest-specific gene 6 expression in human breast cancer. Br. J. Cancer 98, 1141–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiozawa Y., Pedersen E. A., Patel L. R., Ziegler A. M., Havens A. M., Jung Y., Wang J., Zalucha S., Loberg R. D., Pienta K. J., and Taichman R. S. (2010) GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 12, 116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitman S. P., Kohlschmidt J., Maharry K., Volinia S., Mrózek K., Nicolet D., Schwind S., Becker H., Metzeler K. H., Mendler J. H., Eisfeld A. K., Carroll A. J., Powell B. L., Carter T. H., Baer M. R., et al. (2014) GAS6 expression identifies high-risk adult AML patients: potential implications for therapy. Leukemia 28, 1252–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee-Sherick A. B., Eisenman K. M., Sather S., McGranahan A., Armistead P. M., McGary C. S., Hunsucker S. A., Schlegel J., Martinson H., Cannon C., Keating A. K., Earp H. S., Liang X., DeRyckere D., and Graham D. K. (2013) Aberrant Mer receptor tyrosine kinase expression contributes to leukemogenesis in acute myeloid leukemia. Oncogene 32, 5359–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ben-Batalla I., Schultze A., Wroblewski M., Erdmann R., Heuser M., Waizenegger J. S., Riecken K., Binder M., Schewe D., Sawall S., Witzke V., Cubas-Cordova M., Janning M., Wellbrock J., Fehse B., et al. (2013) Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood 122, 2443–2452 [DOI] [PubMed] [Google Scholar]
- 18. Shiozawa Y., Pedersen E. A., and Taichman R. S. (2010) GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B-cell precursor acute lymphoblastic leukemia in the bone marrow niche. Exp. Hematol. 38, 132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caligaris-Cappio F., Bergui L., Gregoretti M. G., Gaidano G., Gaboli M., Schena M., Zallone A. Z., and Marchisio P. C. (1991) Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood 77, 2688–2693 [PubMed] [Google Scholar]
- 20. Kawano Y., Moschetta M., Manier S., Glavey S., Görgün G. T., Roccaro A. M., Anderson K. C., and Ghobrial I. M. (2015) Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 263, 160–172 [DOI] [PubMed] [Google Scholar]
- 21. Noll J. E., Williams S. A., Tong C. M., Wang H., Quach J. M., Purton L. E., Pilkington K., To L. B., Evdokiou A., Gronthos S., and Zannettino A. C. (2014) Myeloma plasma cells alter the bone marrow microenvironment by stimulating the proliferation of mesenchymal stromal cells. Haematologica 99, 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klein B., Lu Z. Y., Gaillard J. P., Harousseau J. L., and Bataille R. (1992) Inhibiting IL-6 in human multiple myeloma. Curr. Top. Microbiol. Immunol. 182, 237–244 [DOI] [PubMed] [Google Scholar]
- 23. Xiao W., Hodge D. R., Wang L., Yang X., Zhang X., and Farrar W. L. (2004) NF-κB activates IL-6 expression through cooperation with c-Jun and IL6-AP1 site, but is independent of its IL6-NFκB regulatory site in autocrine human multiple myeloma cells. Cancer Biol. Ther. 10, 1007–1017 [DOI] [PubMed] [Google Scholar]
- 24. Dankbar B., Padró T., Leo R., Feldmann B., Kropff M., Mesters R. M., Serve H., Berdel W. E., and Kienast J. (2000) Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood 95, 2630–2636 [PubMed] [Google Scholar]
- 25. Platanias L. C. (2003) Map kinase signaling pathways and hematologic malignancies. Blood 101, 4667–4679 [DOI] [PubMed] [Google Scholar]
- 26. Demchenko Y. N., and Kuehl W. M. (2010) A critical role for the NFκkB pathway in multiple myeloma. Oncotarget 1, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tai Y. T., Podar K., Mitsiades N., Lin B., Mitsiades C., Gupta D., Akiyama M., Catley L., Hideshima T., Munshi N. C., Treon S. P., and Anderson K. C. (2003) CD40 induces human multiple myeloma cell migration via phosphatidylinositol 3-kinase/AKT/NF-κB signaling. Blood 101, 2762–2769 [DOI] [PubMed] [Google Scholar]
- 28. Faid L., Van Riet I., De Waele M., Facon T., Schots R., Lacor P., and Van Camp B. (1996) Adhesive interactions between tumour cells and bone marrow stromal elements in human multiple myeloma. Eur. J. Haematol. 57, 349–358 [DOI] [PubMed] [Google Scholar]
- 29. Golub T. R., Slonim D. K., Tamayo P., Huard C., Gaasenbeek M., Mesirov J. P., Coller H., Loh M. L., Downing J. R., Caligiuri M. A., Bloomfield C. D., and Lander E. S. (1999) Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286, 531–537 [DOI] [PubMed] [Google Scholar]
- 30. van 't Veer L. J., Dai H., van de Vijver M. J., He Y. D., Hart A. A., Mao M., Peterse H. L., van der Kooy K., Marton M. J., Witteveen A. T., Schreiber G. J., Kerkhoven R. M., Roberts C., Linsley P. S., Bernards R., and Friend S. H. (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 [DOI] [PubMed] [Google Scholar]
- 31. Linger R. M., Keating A. K., Earp H. S., and Graham D. K. (2008) TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 100, 35–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dirks W., Rome D., Ringel F., Jäger K., MacLeod R. A., and Drexler H. G. (1999) Expression of the growth arrest-specific gene 6 (GAS6) in leukemia and lymphoma cell lines. Leuk. Res. 23, 643–651 [DOI] [PubMed] [Google Scholar]
- 33. Hideshima T., and Anderson K. C. (2002) Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat. Rev. Cancer 2, 927–937 [DOI] [PubMed] [Google Scholar]
- 34. Arnulf B., Lecourt S., Soulier J., Ternaux B., Lacassagne M. N., Crinquette A., Dessoly J., Sciaini A. K., Benbunan M., Chomienne C., Fermand J. P., Marolleau J. P., and Larghero J. (2007) Phenotypic and functional characterization of bone marrow mesenchymal stem cells derived from patients with multiple myeloma. Leukemia 21, 158–163 [DOI] [PubMed] [Google Scholar]
- 35. Wallace S. R., Oken M. M., Lunetta K. L., Panoskaltsis-Mortari A., and Masellis A. M. (2001) Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer 91, 1219–1230 [DOI] [PubMed] [Google Scholar]
- 36. Klein B., Zhang X. G., Jourdan M., Content J., Houssiau F., Aarden L., Piechaczyk M., and Bataille R. (1989) Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood 73, 517–526 [PubMed] [Google Scholar]
- 37. Ramakrishnan V., Kimlinger T., Haug J., Painuly U., Wellik L., Halling T., Rajkumar S. V., and Kumar S. (2012) Anti-myeloma activity of Akt inhibition is linked to the activation status of PI3K/Akt and MEK/ERK pathway. PLoS One 7, e50005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dai Y., Landowski T. H., Rosen S. T., Dent P., and Grant S. (2002) Combined treatment with the checkpoint abrogator UCN-01 and MEK1/2 inhibitors potently induces apoptosis in drug-sensitive and -resistant myeloma cells through an IL-6-independent mechanism. Blood 100, 3333–3343 [DOI] [PubMed] [Google Scholar]
- 39. Chang-Yew Leow C., Gerondakis S., and Spencer A. (2013) MEK inhibitors as a chemotherapeutic intervention in multiple myeloma. Blood Cancer J. 3, e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Q., Tao B., Liu G., Chen G., Zhu Q., Yu Y., Yu Y., and Xiong H. (2016) Thromboxane A2 receptor inhibition suppresses multiple myeloma cell proliferation by inducing p38/c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK)-mediated G2/M progression delay and cell apoptosis. J. Biol. Chem. 291, 4779–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oeckinghaus A., Hayden M. S., and Ghosh S. (2011) Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 [DOI] [PubMed] [Google Scholar]
- 42. Keats J. J., Fonseca R., Chesi M., Schop R., Baker A., Chng W. J., Van Wier S., Tiedemann R., Shi C. X., Sebag M., Braggio E., Henry T., Zhu Y. X., Fogle H., Price-Troska T., et al. (2007) Promiscuous Mutations Activate the Non-Canonical NF-κB Pathway in Multiple Myeloma. Cancer Cell 12, 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fabre C., Mimura N., Bobb K., Kong S. Y., Gorgun G., Cirstea D., Hu Y., Minami J., Ohguchi H., Zhang J., Meshulam J., Carrasco R. D., Tai Y. T., Richardson P. G., Hideshima T., and Anderson K. C. (2012) Dual inhibition of canonical and noncanonical NF-κB pathways demonstrates significant antitumor activities in multiple myeloma. Clin. Cancer Res. 18, 4669–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L. H., Yang X. Y., Zhang X., and Farrar W. L. (2007) Inhibition of adhesive interaction between multiple myeloma and bone marrow stromal cells by PPARγ cross talk with NF-κB and C/EBP. Blood 110, 4373–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mitsiades N., Mitsiades C. S., Poulaki V., Chauhan D., Richardson P. G., Hideshima T., Munshi N., Treon S. P., and Anderson K. C. (2002) Biologic sequelae of nuclear factor-κB blockade in multiple myeloma: therapeutic applications. Blood 99, 4079–4086 [DOI] [PubMed] [Google Scholar]
- 46. Markovina S., Callander N. S., O'Connor S. L., Kim J., Werndli J. E., Raschko M., Leith C. P., Kahl B. S., Kim K., and Miyamoto S. (2008) Bortezomib-resistant nuclear factor-κB activity in multiple myeloma cells. Mol. Cancer Res. 6, 1356–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murray M. Y., Zaitseva L., Auger M. J., Craig J. I., MacEwan D. J., Rushworth S. A., and Bowles K. M. (2015) Ibrutinib inhibits BTK-driven NF-κB p65 activity to overcome bortezomib-resistance in multiple myeloma. Cell Cycle 14, 2367–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Markovina S., Callander N. S., O'Connor S. L., Xu G., Shi Y., Leith C. P., Kim K., Trivedi P., Kim J., Hematti P., and Miyamoto S. (2010) Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-κB activity in myeloma cells. Mol. Cancer 9, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mitsiades C. S., Davies F. E., Laubach J. P., Joshua D., San Miguel J., Anderson K. C., and Richardson P. G. (2011) Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. J. Clin. Oncol. 29, 1916–1923 [DOI] [PubMed] [Google Scholar]
- 50. Hansson M., Gimsing P., Badros A., Niskanen T. M., Nahi H., Offner F., Salomo M., Sonesson E., Mau-Sorensen M., Stenberg Y., Sundberg A., Teige I., Van Droogenbroeck J., Wichert S., Zangari M., et al. (2015) A phase I dose-escalation study of antibody BI-505 in relapsed/refractory multiple myeloma. Clin. Cancer Res. 21, 2730–2736 [DOI] [PubMed] [Google Scholar]
- 51. Muñoz X., Sumoy L., Ramírez-Lorca R., Villar J., de Frutos P. G., and Sala N. (2004) Human vitamin K-dependent GAS6: gene structure, allelic variation, and association with stroke. Hum. Mutat. 23, 506–512 [DOI] [PubMed] [Google Scholar]
- 52. Katagiri M., Hakeda Y., Chikazu D., Ogasawara T., Takato T., Kumegawa M., Nakamura K., and Kawaguchi H. (2001) Mechanism of stimulation of osteoclastic bone resorption through Gas6/Tyro 3, a receptor tyrosine kinase signaling, in mouse osteoclasts. J. Biol. Chem. 276, 7376–7382 [DOI] [PubMed] [Google Scholar]
- 53. Miura A., Honma R., Togashi T., Yanagisawa Y., Ito E., Imai J., Isogai T., Goshima N., Watanabe S., and Nomura N. (2006) Differential responses of normal human coronary artery endothelial cells against multiple cytokines comparatively assessed by gene expression profiles. FEBS Lett. 580, 6871–6879 [DOI] [PubMed] [Google Scholar]
- 54. Ohkawara H., Ishibashi T., Sakamoto T., Sugimoto K., Nagata K., Yokoyama K., Sakamoto N., Kamioka M., Matsuoka I., Fukuhara S., Sugimoto N., Takuwa Y., and Maruyama Y. (2005) Thrombin-induced rapid geranylgeranylation of RhoA as an essential process for RhoA activation in endothelial cells. J. Biol. Chem. 280, 10182–10188 [DOI] [PubMed] [Google Scholar]
- 55. Ohkawara H., Ishibashi T., Sugimoto K., Ikeda K., Ogawa K., and Takeishi Y. (2014) Membrane type 1-matrix metalloproteinase/Akt signaling axis modulates TNF-α-induced procoagulant activity and apoptosis in endothelial cells. PLoS One 9, e105697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harada-Shirado K., Ikeda K., Ogawa K., Ohkawara H., Kimura H., Kai T., Noji H., Morishita S., Komatsu N., and Takeishi Y. (2015) Dysregulation of the MIRLET7/HMGA2 axis with methylation of the CDKN2A promoter in myeloproliferative neoplasms. Br. J. Haematol. 168, 338–349 [DOI] [PubMed] [Google Scholar]