FIGURE 5.
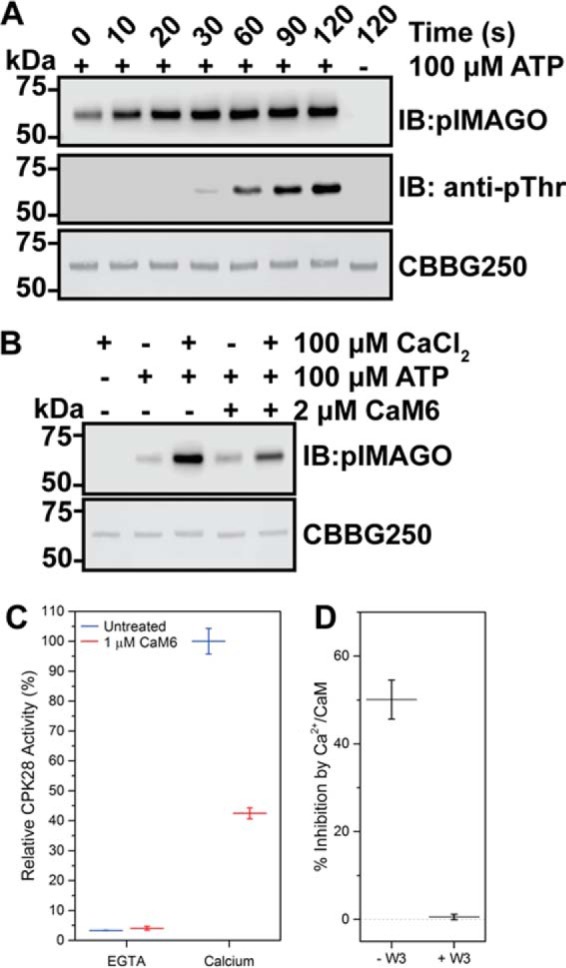
CaM inhibits Ca2+-activated autophosphorylation and transphosphorylation of fully dephosphorylated CPK28. A, time-dependent autophosphorylation of fully dephosphorylated His6-CPK28. A single reaction was initiated by the addition of ATP and was sampled at the time points indicated. A reaction lacking ATP was included as a control. Samples were assessed by blotting with the pIMAGO reagent or with anti-phosphothreonine (anti-pThr) antibodies. Approximately 500 ng of protein was loaded in each lane. B, effect of Ca2+ and Ca2+/CaM on autophosphorylation of CPK28. Reactions were prepared as indicated, initiated by the addition of ATP, and allowed to proceed for 30 s. Samples were assessed by blotting with the pIMAGO reagent, and a CBBG250-stained membrane is shown to demonstrate equal loading (∼500 ng) for each sample. C, effect of Ca2+ and Ca2+/CaM on CPK28 peptide kinase activity. CPK28 activity toward the ACSM+1 peptide (10 μm) was assessed in the absence (EGTA) or presence (calcium) of 100 μm CaCl2 with or without the addition of 1 μm CaM6 as indicated. Values reported are CPK28 activity relative to the Ca2+-activated state. D, W3 peptide blocks Ca2+/CaM inhibition of CPK28 peptide kinase activity. Activity of Ca2+-activated CPK28 was assessed in the absence (− W3) or presence (+ W3) of 2 μm W3 peptide, with or without the addition of 1 μm CaM6 as indicated. Specific activities under each condition tested are shown (inset). Point estimates in C and D represent mean with standard deviation of three technical replicates. Data shown are representative of at least two independent preparations of recombinant CPK28. IB, immunoblot; CBBG250, Coomassie Brilliant Blue G-250.
