FIGURE 9.
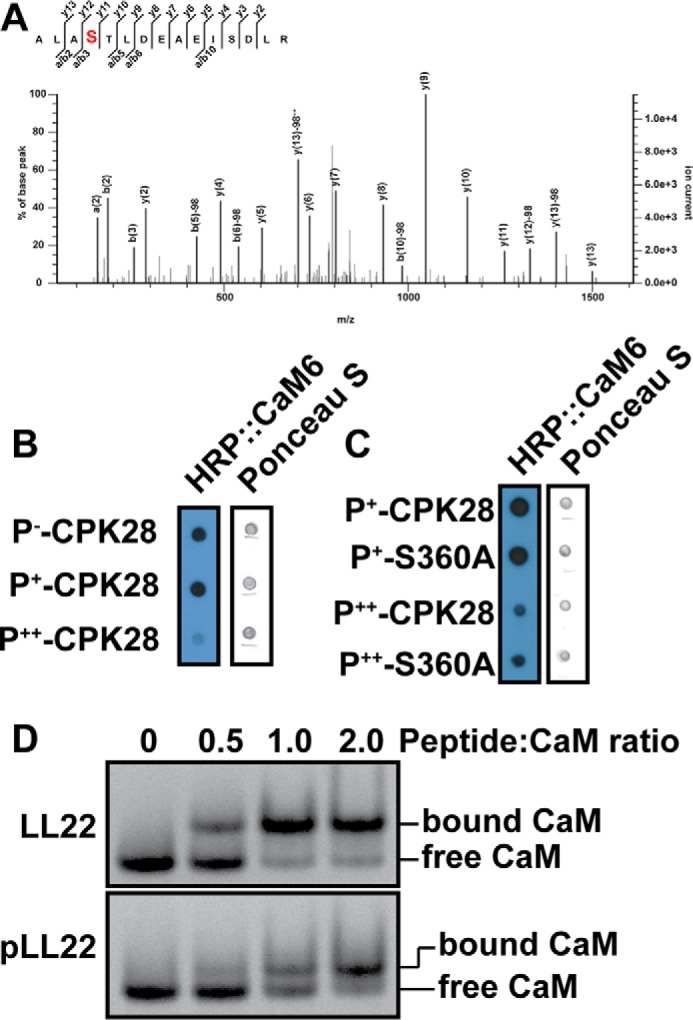
Effect of Ser-360 autophosphorylation on CaM binding to CPK28. A, product ion spectrum identifying Ser-360 within the CPK28 CaM-binding domain as an autophosphorylation site. B, HRP::CaM6 binding to the three CPK28 phosphoforms. Approximately 400 ng of each protein was spotted, and the blot was probed with 100 nm HRP::CaM6 in the presence of calcium (2 mm). C, HRP::CaM6 binding for phospho-CPK28 and S360A mutants. Approximately 400 ng of each protein was spotted, and the blot was probed with 100 nm HRP::CaM6. D, native PAGE analysis of CaM binding to the LL22 peptide and its phosphorylated variant (pLL22). A near-complete mobility shift of CaM required more pLL22 peptide compared with the dephosphorylated form. CaM (240 pmol) was incubated with different amounts of peptide in the ratios indicated in a 20-μl reaction for 60 min prior to electrophoresis. HRP, horseradish peroxidase.
