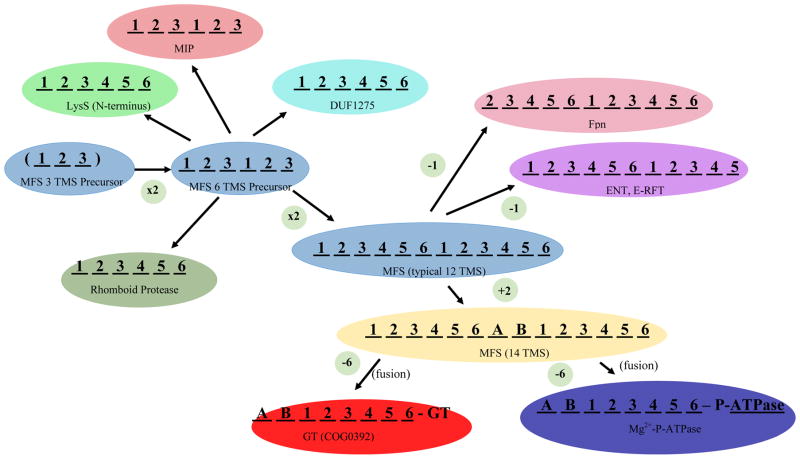
Figure 3.
The proposed precursor for all members of the MFS was a 3 TMS peptide (blue; center left), possibly derived from a 2 TMS plus P-loop voltage-gated ion channel [31] (see text). This 3 TMS unit duplicated twice, first to a 6 TMS protein, and then again to give a 12 TMS protein, characteristic of most MFS carriers (TC# 2.A.1) (light blue; central left and middle). In a few cases, 12 TMS MFS carriers have duplicated or fused to give 24 TMS proteins catalyzing related but different transport processes (not shown). Six TMS products of the proposed 3 TMS duplication also gave rise to members of the MIP family of aquaporins and glycerol facilitators (TC# 1.A.8) (orange; upper left), the N-terminal 6 TMS domains of LysS family members (Lysyl-tRNA syntheases; TC# 9.B.111) (light green; upper left) proteins of the DUF1275 family (TC# 9.B.143) of unknown function (dark green; upper center), and the 6 TMS Rhomboid proteases (pea green; lower left). The basic 12 TMS MFS backbone lost an N-terminal TMS (Ferroportin (Fpn) Family; TC# 2.A.100; pink, upper right), or a C-terminal TMS (the ENT and E-RFT families, TC# 2.A.57 and 2.A.125, respectively) (purple; upper right). Two TMSs (A and B), found in several MFS families, were inserted between the two 6 TMS repeat units. TMSs A and B are believed to have arisen independently in several different MFS families, possibly by intragenic duplication of the adjacent α-helical hairpin-encoding genetic element (yellow; lower right) [30]. From the last 8 TMSs of a 14 TMS protein, possibly of the MFS family, DHA2 (TC# 2.A.1.3), transmembrane regions of glycosyl transferases (GT) (red; bottom center) and the N-terminal domains of some P-type Mg2+-transporting ATPases (TC# 3.A.3.4) (dark blue; bottom left) arose.