Figure 2.
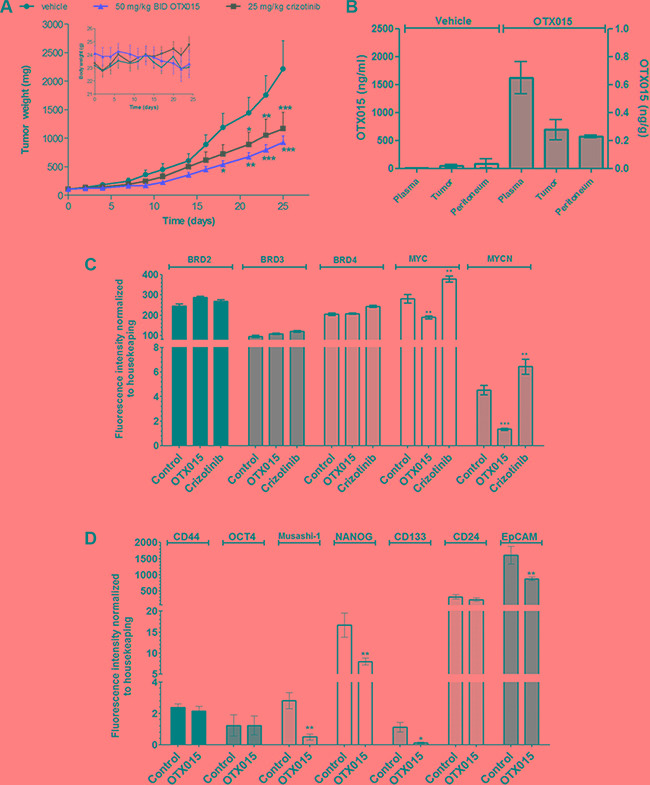
(A) OTX015 in vivo effects in NSLCL-ALK(+) H3122-tumor bearing mice. Antitumor effects of 50 mg/kg OTX015, BID, 7 days ON, gavage or 25 mg/kg crizotinib, thrice weekly, gavage were compared with vehicle thrice weekly, gavage in H3122 murine xenografts (n = 10 mice per group). Asterisks indicate significant differences in tumor mass between each single agent arm versus the vehicle-treated group (*p < 0.05, **p <0.01 and ***p < 0.001). (B) OTX015 concentrations in plasma and tissue from H3122 xenografts. OTX015 levels were evaluated in terminal plasma, peritumoral normal and tumor tissue from H3122-bearing mice treated with 50 mg/kg OTX015 BID or vehicle for 25 days. Mice were sacrificed 4 h after the last administration. Results are expressed as mean ± SD. (C) Gene expression of BRDs and MYC family genes by qPCR in H3122 tumors after treatment with OTX015. Differences in gene expression between OTX015 (50 mg/kg OTX015) and crizotinib (25 mg/kg thrice weekly) with respect to controls were evaluated with one-way Anova followed by Dunnett's Multiple Comparison Test (**p < 0.01 and ***p < 0.001). (D) Gene expression of stem cell markers by qPCR in H3122 tumors after treatment with OTX015. Differences in gene expression between OTX015 and crizotinib with respect to controls were evaluated with one-way Anova followed by Dunnett's Multiple Comparison Test (*p < 0.05 and **p < 0.01).
