Figure 5. The effects of baicalein on E2-induced ERα nuclear transcriptional activation.
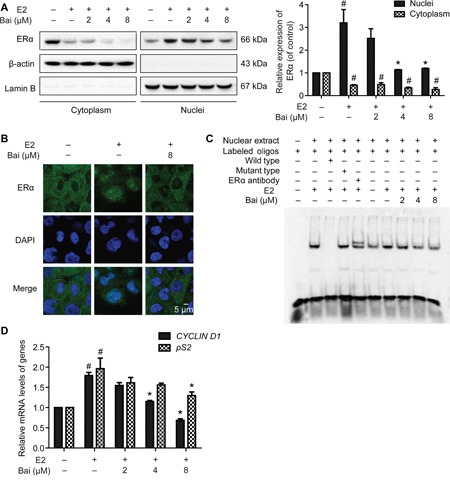
After pre-incubation in serum-free medium for 24 h, the cells were treated with E2 or E2 plus baicalein under the same condition for 24 h. A. The localization of ERα in cytoplasmic and nuclear fractions was analyzed by western blotting. The cytoplasmic and nuclear levels of ERα were normalized to β-actin and lamin B, respectively. B. The subcellular localization of ERα was confirmed by confocal microscopy. Images of ERα (green fluorescence) and cell nuclei stained with DAPI (blue fluorescence) are shown (×1000). Scale bar = 5 μm. C. EMSA was used to detect the binding of ERα to estrogen response elements (EREs). The composition of the DNA-binding complex was investigated using anti-ERα antibodies in supershift experiments. The concentration of the unlabeled wild-type or mutant probes (as competitor oligonucleotides) was 100-times greater than that of the labeled wild-type probe. D. The effects of baicalein on E2-induced ERα-regulated gene expression were analyzed using RT-PCR. The results obtained from experiments were normalized to GAPDH expression and are shown as the fold-change compared with control cells. Data are shown as means ± SEM (n = 3). *P < 0.05 vs. E2, #P < 0.05 vs. control.
