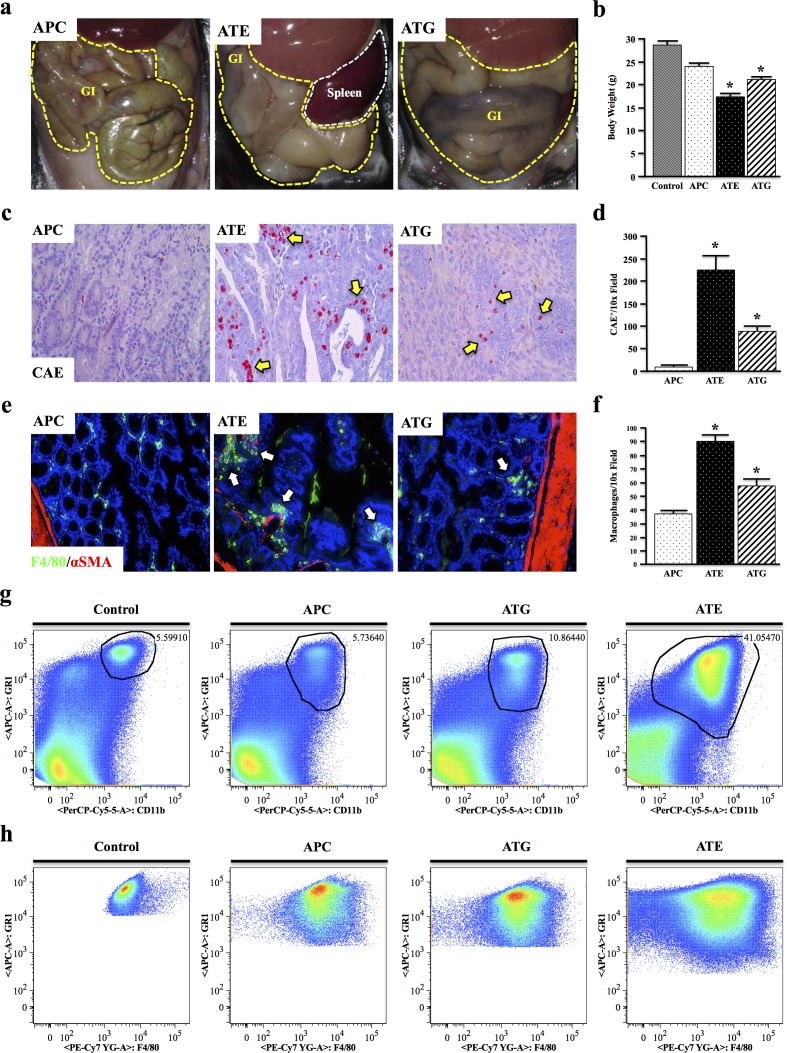
Figure 3. Both Systemic and Epithelial TGFBR-Deficiency Enhance APC-Induced Inflammation.
a. At four months, ATE and ATG mice presented with severely distended GI tracts compared to APC mice. b. ATE and ATG mice were also grossly underweight, weighing on average ~60% of control mice. c.-d. Mouse tissues were subject to Chloracetate Esterase (CAE) staining, which indicated a near 26-fold increase in the number of inflammatory granulocytes in the colon of ATE mice compared to APC controls, and a near 2.5-fold increase compared to ATG. e.-f. Staining for the mouse macrophage marker F4/80, which showed an increase in tumor infiltrating macrophages in TGFBR-deficient mice, most notably in ATE, localizing predominantly to the colon stroma. g. Spleens were harvested from nongenic control, APC, ATG, and ATE animals, and analyzed for expression of the myeloid marker CD11b and GR1, a marker of most inflammatory granulocytes. While ATG animals displayed an approximate two-fold increase in GR1+CD11b+ cells, ATE mice had a 7.33-fold increase over control mice, consistent with a more severe inflammatory phenotype. h. Gating to GR1+CD11b+ populations in control and mouse models revealed strong positivity for the macrophage marker F4/80. (*, P < 0.05. N = 4 per group). It should be noted that, due to the large differences in the size and location of the population of interest, it was necessary to gate manually.