Abstract
Background
Adhesion G protein-coupled receptors (aGPCRs) are a large family of transmembrane proteins that play important roles in many processes during development, primarily through cell-cell and cell-extracellular matrix (ECM) interactions. In the nervous system, they have been linked to the complex process of myelination, both in the central and peripheral nervous system.
Results
GPR126 is essential in Schwann cell-mediated myelination in the peripheral nervous system (PNS), while GPR56 is involved in oligodendrocyte development central nervous system (CNS) myelination. VLGR1 is another aGPCR that is associated with the expression of myelin-associated glycoprotein (MAG) which has inhibitory effects on the process of nerve repair. The ECM is composed of a vast array of structural proteins, three of which interact specifically with aGPCRs: collagen III/GPR56, collagen IV/GPR126, and laminin-211/GPR126.
Conclusions
As druggable targets, aGPCRs are valuable in their ability to unlock treatment for a wide variety of currently debilitating myelin disorders.
Keywords: development, signaling, injury response
Introduction
Myelination is a complex process that begins in the postnatal period and lasts to adulthood, in both the peripheral and central nervous system, involving the interplay of several key characters. Myelin is the fatty membrane of glia cells, high in cholesterol and phospholipid content, that wraps around the axons of neurons in both the central nervous system (CNS) and peripheral nervous system (PNS), to allow for rapid salutatory conduction of nerve impulses and to contribute to axonal integrity and health (Domingues et al., 2016). While the function of myelin is similar within the two systems, the composition of myelin varies between the CNS and PNS, with proteolipid protein (PLP) as the dominant protein of CNS myelin and myelin protein zero (P0) as the major component in PNS myelin (Nave and Werner, 2014). The process of myelination also differs between the CNS and PNS, mediated by either oligodendrocytes (OL) or Schwann cells (SC), respectively.
When these processes are disrupted, it leads to diseases of varying severity. These can include neuropathies, which can be congenital or acquired leukodystrophies resulting from mutations in any number of genes involved throughout the myelination process, and chronic demyelination diseases such as multiple sclerosis, in which ongoing neuroinflammation and myelin destruction coupled with inadequate repair process remain a significant aspect of the disease (Nave and Werner, 2014). Nerve injury leading to demyelination, whether mechanical or through disease, invokes a complicated process of axonal regeneration and remyelination. Just as with myelin formation, the repair processes are mediated by cells of the oligodendrocyte lineage in the CNS and Schwann cell lineage in the PNS.
Adhesion G-protein Coupled Receptors
Adhesion G-protein coupled receptors (aGPCRs) comprise the second largest class of GPCRs that play important roles in many developmental processes (Langenhan et al., 2013; Hamann et al., 2015; Langenhan et al., 2016). There are a total of 33 members of aGPCRs in the human genome. Structurally, aGPCRs are characterized by an extremely large extracellular N-terminal region and a 7-transmembrane domain that facilitates downstream signaling, both G-protein dependent and G-protein independent signaling. Most aGPCRs undergo GPCR Autoproteolysis INducing (GAIN) domain-mediated autoproteolytic process to generate an N- and a C-terminal fragment, NTF and CTF, respectively. The NTF and CTF remain non-covalently associated on the cell surface. By virtue of the large NTF, aGPCRs are often involved in cell-cell and cell-extracellular matrix interactions. Upon ligand binding, the NTF is removed from its CTF, revealing a tethered agonist that activates downstream G-protein signaling (Liebscher et al., 2014; Petersen et al., 2015; Stoveken et al., 2015) (Fig. 1). Because of their interactions with adjacent cells and the extracellular matrix and their ability to have numerous binding partners, aGPCRs often play important roles in complex signaling pathways, including those in development and repair. Most aGPCRs are “orphan receptors” with unknown ligands. Given that a large percentage of today’s pharmaceuticals target GPCRs, the importance of identifying ligands for these receptors cannot be overestimated.
Figure 1.
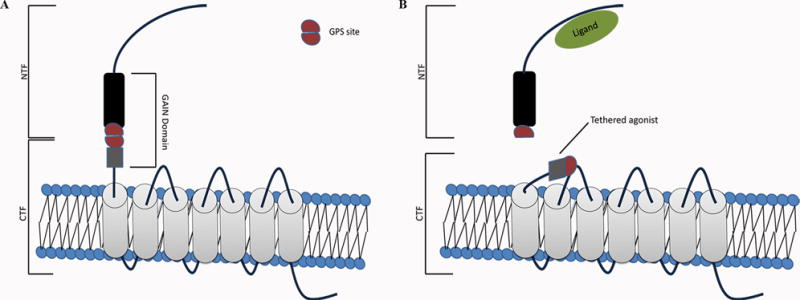
Cartoon depicting the general structure and signaling mechanism for aGPCRs. A) General structure of aGPCRs. Adhesion GPCRs are characterized by their extremely long N-terminal fragment (NTF) that contains a GAIN domain, within which is the site of autoproteolysis (GPS). The C-terminal fragment (CTF) contains a 7-transmembrane domain (7-TM). After autoproteolysis, the NTF and the CTF remain covalently associated at the cell surface. B) Tethered agonist activation. Upon ligand binding, the NTF detaches from the CTF and uncovers the tethered agonist peptide signal, which interacts with the 7-TM and facilitates downstream G-protein signaling.
In this review, we discuss the interplay between aGPCRs and the extracellular matrix of the central and peripheral nervous system throughout the processes of myelin formation and repair.
aGPCRs and Myelination in the CNS
In the CNS, oligodendrocyte precursor cells (OPCs) proliferate and terminally differentiate to generate mature oligodendrocytes (Emery, 2010). OLs initiate myelination by extending their processes until they make contact with an axonal membrane, which then induces a number of molecular and biochemical changes(Baer et al., 2009) and the reorganization of cytoskeletal elements (Nave and Werner, 2014). Mature OLs complete myelination by enwrapping neuronal axons and then provide trophic support to axons (Funfschilling et al., 2012). In the setting of nerve injury in the CNS, remyelination is regulated by the recruitment of OL precursor cells (OPCs) and subsequent differentiation into mature myelinating OLs, as well as the mobilization of adult neural stem cells through inhibition of Gli1, a transcriptional effector of the sonic hedgehog pathway (Ransohoff, 2012; Franklin and Gallo, 2014; Samanta et al., 2015; Domingues et al., 2016). Evidence shows that OPCs constantly proliferate in the CNS to maintain their cell density (Hughes et al., 2013), in order to continue producing and remodeling myelin throughout adulthood, an important mechanism for neuroplasticity (McKenzie et al., 2014; Yeung et al., 2014). In the setting of nerve injury in the CNS, mature OLs degenerate and die (active demyelination), and nearby OPCs need to be “activated” by the surrounding environment, allowing for proliferation and mobilization to the site of injury, accompanied by increased expression of genes associated with differentiation (Fancy et al., 2004; Domingues et al., 2016).
GPR56/ADGRG1
aGPCR GPR56 was first cloned by two independent groups, one searching for further members of the secretin family of GPCRs and the other screening for differentially expressed genes in a human melanoma metastasis model (Liu et al., 1999; Zendman et al., 1999). However, the function of GPR56 was not known until 2004 when its mutations were linked to the development of bilateral frontoparietal polymicrogyria (BFPP) (Piao et al., 2004). BFPP is an autosomal recessive disorder characterized by multiple small gyri and abnormal cortical lamination, primarily in the frontoparietal regions of the brain (Piao et al., 2004; Piao et al., 2005). In Gpr56 knockout mice, there is disruption of normal neuronal migration, with a breach of the pial basement membranes by overmigrating neurons (Li et al., 2008). This finding was subsequently confirmed in post-mortem BFPP brains (Bahi-Buisson et al., 2010).
In addition to the cortical defects, radiological features of BFPP patients also include thinned white matter with areas of T2 prolongation on MRI, suggesting that GPR56 may play a role not only in neuronal migration but also in the process of myelination (Chang et al., 2003; Piao et al., 2004; Piao et al., 2005). Deleting Gpr56 leads to CNS hypomyelination in both mice and zebrafish (decreased number of myelinated axons with preserved myelin thickness) and a decreased number of mature oligodendrocytes (Ackerman et al., 2015; Giera et al., 2015), suggesting an evolutionarily conserved function of GPR56 in OL development and CNS myelination. In mice, this disparity in myelination status was erased by 6 months post-natal age, suggesting correction by ongoing oligodendrocyte production. To determine whether the decreased number of mature OLs is secondary to decreased production versus impaired OL differentiation/maturation, Gpr56 expression was determined along the OL lineage. Expression of Gpr56 was found primarily in glial cell progenitors and most OPCs (~80%) and down-regulated in mature myelinating OLs (Fig. 2A). It is possible that the 20% GPR56 negative OPCs account for the eventual full myelination observed in Gpr56 knockout mice by 6 months of age (Giera et al., 2015). GPR56 regulates OPC proliferation through Gα12/13 and RhoA downstream signaling (Fig. 2B). In the absence of Gpr56, OPCs exit the cell cycle prematurely, resulting in a decreased progenitor pool and reduced number of mature OLs (Giera et al., 2015). Deletion of Gpr56 does not affect the terminal differentiation of OPCs (Giera et al., 2015).
Figure 2.
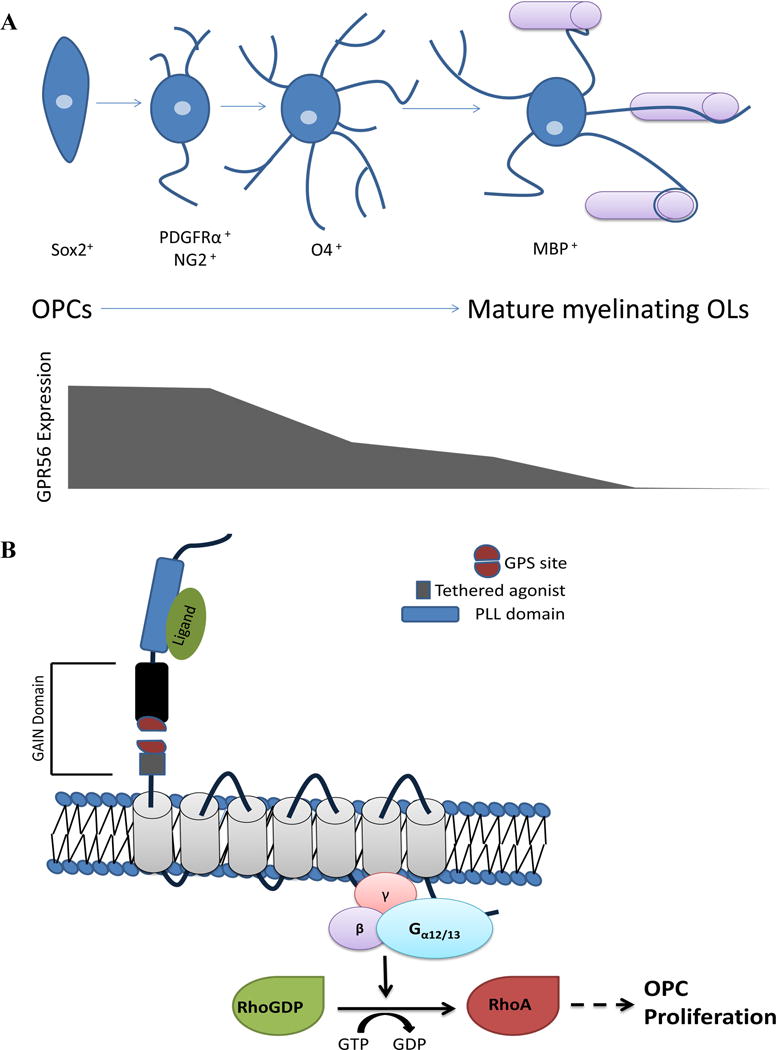
GPR56 promotes OPC proliferation. A) As OPCs progress through differentiation, they begin to extend their processes and create complex branching structures, with the mature myelinating OLs wrapping their processes around axons to create a myelin sheath. GPR56 is highly expressed in glial progenitors and OPCs, but is downregulated in O4+ immature OLs, with little to no expression noted by mature OLs. B) GPR56 binds an unknown ligand that leads to downstream RhoA activation through coupling to Gα12/13. RhoA activation then leads to OPC proliferation. PLL, Pentraxin/Laminin/neurexin/sex-hormone-binding-globulin-like domain.
The ligand of OL GPR56 is unknown. To date, GPR56 has been shown to bind multiple partners. Collagen III is the ligand of GPR56 in the developing cerebral cortex (Luo et al., 2011b). The binding of collagen III and GPR56 inhibits neural migration through coupling to Gα12/13 and activates RhoA pathway (Luo et al., 2011a). Besides meningeal fibroblasts, collagen III is also expressed in the endothelium of blood vessels in the developing brain. As OPCs use CNS vasculature to navigate their migration, it is pertinent to consider Col III as a GPR56 ligand during OL development. In a melanoma model, GPR56 binds transglumatinase (TG2), a major crosslinking enzyme in the ECM that regulates cell adhesion and leads to activation of protein kinase C (Xu et al., 2006). TG2 has been shown to play a prominent role in the remyelination of the CNS, (Van Strien et al., 2011). However, the interaction between GPR56 and TG2 in the CNS has not yet been fully defined. In an in vitro culture system, the NTF of GPR56 was found to bind heparin, where heparin binding reduces GPR56 receptor shedding without affecting membrane distribution of either the NTF or CTF (Chiang et al., 2016). Noting that both known ligands of GPR56, collagen III and TG2, are heparin-binding proteins, it is possible that heparin may influence how GPR56 interacts with its other protein ligands.
Recently, the NTF of GPR56 was crystallized, the first full crystal structure of the NTF of any aGPCR (Salzman et al., 2016). This 3-D model allowed for discovery of a number of previously unknown aspects to the structure of GPR56. The NTF is made of two domains linked together using a disulfide bond. The first, known as the Pentraxin/Laminin/neurexin/sex-hormone-binding-globulin-like (PLL) domain, is comprised of a 12-sheet β sandwich with weak homology to pentraxin and laminin/neurexin/sex hormone-binding globulin domain families. The second is the GAIN domain, which is smaller than other known GAIN domains in aGPCRS but retains autoproteolysis ability (Salzman et al., 2016). There are four different isoforms of GPR56, one of which, the splice variant 4 (S4), uses an alternative starting ATG for translation in exon 4 and creates a protein lacking the PLL domain, suggesting that the presence or absence of PLL may be a method in which to diversify functionality of GPR56 in vivo (Salzman et al., 2016). In fact, isoforms lacking the PLL have increased basal activity of GPR56 (measured through a SRE-luciferase assay). Point mutation analyses in zebrafish revealed a conserved patch within the PLL domain that appears to mediate OL development (some mutants found to have decreased mbp expression; others resulted in overexpression of mbp) (Salzman et al., 2016). This patch may directly engage GPR56 ligand binding since binding sites for TG2 and collagen III are both located within the PLL domain (Yang et al., 2011; Luo et al., 2012).
Besides exerting its function in OL lineage, GPR56 likely plays an indispensable role in other glial cell populations in the postnatal brain. A recent gene profiling from different cell types in mouse neonatal brains revealed a high Gpr56 transcript level in microglia (Zhang et al., 2014). Microglia are the innate immune cells of the nervous system and have been implicated throughout the processes of myelination and nerve repair. They act as sensors of pathologic events, and their activation has been extensively described in autoimmune disease models such as the mouse model of multiple sclerosis (Domingues et al., 2016; Ransohoff, 2016). Through an increase of the PDGF-α receptor signaling pathway and regulation of NF-κB activation, non-activated microglia were able to enhance OPC survival and maturation (Nicholas et al., 2001). In rodent models, activated microglia were shown to stimulate OL differentiation (Pasquini et al., 2011; Miron et al., 2013; Shigemoto-Mogami et al., 2014). While the exact role of GPR56 in microglia has not yet been fully explored, the high level of expression suggests that it may play an important role in overall microglial function.
VLGR1/ADGRV1
Another aGPCR, VLGR1 (also referred to by ADGRV1, GPR98, MASS1), was first linked to audiogenic seizures in a mouse model and has been found to be linked to Usher syndrome, an autosomal recessive disorder characterized by hearing loss, retinitis pigmentosa, and vestibular dysfunction (Weston et al., 2004; Hilgert, 2009; Moteki et al., 2015; Yang et al., 2016). Expressed on mature OLs, VLGR1 is also associated with expression of myelin-associated glycoprotein (MAG) (Shin et al., 2013), a cell membrane glycoprotein identified as an inhibitor of axon growth in the CNS after injury (McKerracher et al., 1994; DeBellard et al., 1996; Li et al., 1996). MAG is found to be enriched at peri-axonal regions of myelin sheaths in both the peripheral and central nervous system (Roda et al., 2016). Knockdown of Vlgr1 leads to reduced MAG expression, partly through absent posttranslational regulation as well as decreased slowing of proteasomal degradation (Shin et al., 2013). Signaling studies of VLGR1 reveals that downstream signaling occurs through Gαs/Gαq, activating PKA and protein kinase C (PKC) respectively. It is through this mechanism that post-translational regulation of MAG occurs. Work has shown that elevating cellular levels of cyclic AMP (which activates PKA) can block the inhibitory effects of MAG in the setting of nerve injury and perhaps lead to increased axonal regeneration (He et al., 2016).
aGPCRs and Myelination/Nerve Repair in the Peripheral Nervous System
In the PNS, Schwann cells (SC) are the primary cells responsible for myelination. Immature SCs initially associate with multiple axons, and then, through a process known as radial sorting, select individual axons for myelination. As SCs mature, those that perform radial sorting are pro-myelinating and generate the myelin sheath around the chosen axons. Other SCs mature into non-myelinating Remak SCs that ensheath the “Remak bundle” of non-myelinated axons, composed of multiple small-caliber axons (Monk et al., 2015) (Fig. 3A). Nerve damage leads to degeneration of the distal axons and destruction of myelin sheaths within the SC cytoplasm, a process called Wallerian degeneration (Jang et al., 2016). Nerve regeneration depends on the presence of living SCs distal to the site of injury (Jessen et al., 2015). The SCs rapidly go through a number of changes, the first of which involves de-differentiation out of a myelin maintenance phase – myelin-associated genes are downregulated and markers that characterize immature SCs are upregulated. The SCs then transform into a “repair” state, distinct from immature SCs or those associated with normal myelinated nerves. These “Bungner” cells upregulate the expression of cytokines and activate an autophagy-mediated process for myelin degradation. These cytokines function to recruit macrophages to the area of injury, promote axonal regeneration by acting directly on neurons, and also promote vascularization of the distal nerve, allowing for a more robust repair. Bungner SCs also form Bungner bands, which are columns of cells that act as a regenerative “track” from the area of injury to nerve target areas, guiding regenerating axons (Jessen et al., 2015). Activation of autophagolysosomes in the SC leads to “myelinophagy”, in which myelin debris is present in autophagosomes and inhibition of autophagy (either pharmacological or genetic) leads to impaired myelin clearance (Gomez-Sanchez et al., 2015).
Figure 3.
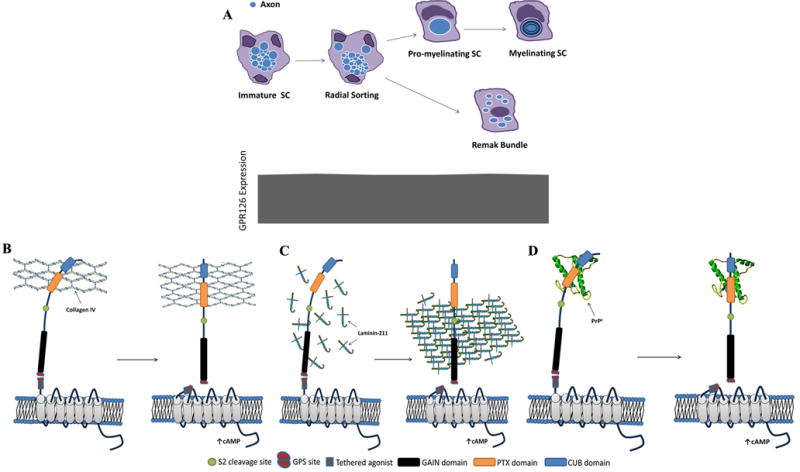
GPR126 regulates both radial sorting and myelination. A) Immature SCs migrate through the PNS, enveloping several axons before undergoing radial sorting and then proceeding down one of two distinct pathways. The first pathway involves the SC selecting and ensheathing a single axon (as a promyelinating SC) and then continuing on to myelinate that specific axon. The second involves becoming a non-myelinating SC that creates “Remak bundles” of non-myelinated axons. These Remak cells also play important roles in repair of myelin damage. The developmental expression of GPR126 is not known. However, current literature suggests that GPR126 is expressed in both immature and mature SCs. Loss of GPR126 leads to a breakdown in both pathways, with SCs arrested in the promyelinating phase, as well as decreased numbers of non-myelinating SCs. B) Collagen IV binds to GPR126 in the region of the CUB-PTX domains which leads to elevation of cAMP. C) In the beginning stages of development, laminin-211 exists as monomers and does not have sufficient interaction with GPR126 to stimulate downstream cAMP signaling. As the basal lamina matures, laminin-211 self-polymerizes and generates adequate force to facilitate GPR126 tethered agonist mediated signaling, increasing downstream cAMP and initiating myelin formation. D) The most recently discovered ligand of GPR126 is PrPc, a prion protein, that has an amino-terminal flexible tail that contains a cAMP inducing domain similar to that found on collagen IV. Application of this moiety was enough to induce a GPR126-dependent cAMP response.
CUB, C1r/C1s domain; PTX, pentraxin domain.
GPR126/ADGRG6
GPR126 is an aGPCR discovered in the early 2000s after a search through the human genome database (Fredriksson et al., 2003a; Fredriksson et al., 2003b; Bjarnadottir et al., 2004). Its integral function in SC development and PNS myelination was discovered through a forward genetic screen in zebrafish (Monk et al., 2009). GPR126 triggers myelination by increasing cyclic AMP (cAMP)/activating protein kinase A (PKA) through Gαs protein signaling, initiating a transcription factor cascade including Oct6 and Krox20, factors essential for SC terminal differentiation and myelination (Monk et al., 2011; Glenn and Talbot, 2013; Mogha et al., 2013). In a knockout mouse model, complete absence of GPR126 led to radial sorting defects, PNS myelination impairment (CNS myelination, quality and amount, was unaffected), and limb contracture defects (Monk et al., 2011) (Fig. 3A). The SCs were arrested at the promyelinating phase and did not express MBP or P0, and there were no signs of developing Remak bundles, despite the presence of small caliber C-fiber axons (axons normally ensheathed by Remak SCs). In addition, overall axon number was markedly reduced in knockout mice, suggesting a role for GPR126 in axon maintenance/trophic support by SCs (Monk et al., 2011). Mutant zebrafish also displayed similar phenotypes, with arrest of SCs in the promyelinating phase and no discernible MBP expression in the PNS (Monk et al., 2009).
In humans, mutations in Gpr126 have been linked with arthrogryposis multiplex congenita (Ravenscroft et al., 2015) and genetic polymorphisms have been linked with adolescent idiopathic scoliosis (Kou et al., 2013; Karner et al., 2015; Xu et al., 2015). Patients with arthrogryposis multiplex congenital are noted to have hypomyelinated nerve fibers on biopsy, underscoring the evolutionarily conserved nature of GPR126 (Ravenscroft et al., 2015).
As with majority of aGPCRs, GPR126 undergoes autoproteolytic cleavage into an NTF and a CTF that have been shown to have distinct functions in the development and function of Schwann cells. The NTF is independently sufficient and essential for radial sorting (Petersen et al., 2015). In a zebrafish model with an intact NTF and absent CTF, radial sorting is preserved while myelination is impaired (Monk et al., 2011), whereas a zebrafish mutant in which an early stop-codon leading to absence of both NTF and CTF demonstrates severe disturbances in radial sorting and a near complete-absence of myelination (Petersen et al., 2015). This phenotype was not rescued by elevating levels of cAMP (which induces expression of MBP in CTF-intact Gpr126 mutants), further confirming that the NTF alone drives radial sorting. The exact mechanism through which the NTF drives radial sorting remains to be discovered.
Collagen IV, a component of the SC basal lamina, was first found as an endogenous ligand that induces cAMP signaling in GPR126-intact SCs. It binds to the region of the NTF containing the CUB/PTX domains, which are conserved domains that bind to other collagen-like proteins in a variety of proteins (Paavola et al., 2014). The binding of collagen IV to GPR126 stimulates the production of cAMP, which combined with the observation that constructs containing the intact CTF alone are constitutively active, suggesting that the NTF antagonizes receptor signaling activity with ligand-binding relieving this repression (Paavola et al., 2014) (Fig. 3B).
Laminin-211, another essential component of the SC basal lamina, also has important interactions with GPR126. It binds the GAIN domain on the NTF, distinct from the domain that binds collagen IV. The binding of laminin-211 to GPR126 triggers a completely different downstream signaling response. Under static conditions, administration of laminin-211 results in a concentration-dependent decrease of cAMP accumulation, whereas applying vibration forces leads to elevated cAMP concentration (Petersen et al., 2015). It is possible that laminin-211 may facilitate physical removal of the NTF, under dynamic condition, to facilitate tethered-agonist mediated activation upon mechanical stimulation (such as stiffening of the extracellular matrix) and holds GPR126 in an inactive state under static conditions. Indeed, polymerization of laminin-α2 chain is required for GPR126-mediated myelination, implying it may be the in vivo mechanical force involved in the removal of the NTF upon laminin-211 binding (Petersen et al., 2015) (Fig. 3C).
A third agonist has been recently discovered, while investigating a chronic demyelinating neuropathy model affecting SCs. Prion diseases occur when a normal cell-surface glycoprotein (PrPc) is conformationally altered into a pathogenic isoform (PrPSc), resulting in transmissible neurodegenerative diseases (Westergard et al., 2007). Prior studies have shown that ablation of PrPc triggers a late-onset chronic demyelinating polyneuropathy in mouse models, indicative of impaired axon-SC interactions. These studies also revealed that in this disease model, PrPc is of primarily neuronal origin (not SCs), implying that PrPc is a crucial neuronal mediator of peripheral myelin maintenance and that SCs bear a PrPc receptor (Bremer et al., 2010). Application of PrPc on SCs induces a concentration-dependent increase in cAMP, which was not seen in cells in which GPR126 was ablated (or in cells expressing a number of other GPCRs) (Kuffer et al., 2016). Further investigation into the structure of PrPc reveals that its amino-terminal flexible tail shares a cAMP-inducing domain with collagen IV, a known agonist of GPR126. A peptide containing this motif alone was sufficient to induce a GPR126-dependent cAMP response (Kuffer et al., 2016) (Fig. 3D). Given the late-onset of the PrPc phenotype, this suggests that the GPR126- PrPc interaction is important for myelin homeostasis.
Frequently, tissue repair recapitulates development. As GPR126 is essential for the development of Schwann cells, it likely also plays a significant role in the repair mechanisms found after PNS injury. Supporting this hypothesis is the recent discovery that the presence of laminin proteins in the basal lamina helps to increase production of neutrophic factors in the setting of nerve injury, suggesting that this relationship helps create the appropriate microenvironment for repair in degenerative disease states (Zarinfard et al., 2016).
Extracellular Matrix Proteins in Myelination and Nerve Repair
The extracellular matrix (ECM) is the non-cellular component throughout the body that provides not only physical scaffolding but also a variety of substrates needed for signaling, metabolism, and homeostasis (Frantz et al., 2010). Each organ system has an ECM with a unique composition, targeted towards the needs of those specific functions. The primary components of ECMs are proteoglycans and fibrous proteins, which include collagens, elastins, fibronectins, and laminins (Frantz et al., 2010). In the PNS, adult nerve fibers are protected by the endoneurium, a dense ECM which is collagen-rich and vascularized, that is linked to the basal lamina produced by and associated with Schwann cells (Jessen et al., 2015). In the CNS, the traditional basal lamina is only found lining the endothelial cells and pial surfaces; the parenchymal ECM components are found in the form of dense networks (Rauch, 2007).
As noted previously, three known ECM proteins have significant relationships with aGPCRs in both CNS and PNS: collagen III/GPR56, laminin-211/GPR126, collagen IV/GPR126. Even aside from their interactions with aGPCRs, families of ECM proteins have aGPCR-independent roles in myelination and nerve repair.
The collagen family makes up a major component of the ECM, especially in the PNS. Ascorbic acid, essential for post-transcriptional modification of collagen polymers, promotes the deposition of Schwann cell ECM in culture and is essential for in vitro myelination (Eldridge et al., 1987). Schwann cells also express several types of collagen molecules as well as their receptors (Chen et al., 2015a). Collagen IV not only functions as a binding partner for GPR126 and activates its downstream signaling, but also promotes the attachment/spreading of SCs and enhances their proliferation through interactions with integrin α1β1 and α2β1 (Lein et al., 1991; Detrait et al., 1999). p200, a novel isoform of collagen V, has been shown to promote SC adhesion, spreading, and migration (Chen et al., 2015a). siRNA-mediated suppression of p200 also significantly inhibits SC myelination in vitro, indicating its likely important role in regulation of in vivo myelination (Chernousov et al., 2006).
Collagen VI is highly expressed by mature SCs but not immature SCs, and the activation of transcription of collagen VI genes is part of the differential program of SCs during maturation (Chen et al., 2015a). In collagen VI knockout mice, adult mice sciatic nerves display significant hypermyelination, suggesting that collagen VI plays an inhibitory role in the myelination process (Chen et al., 2014). These mice also display impaired nerve regeneration in the setting of injury, owing to a failure of recruiting macrophages to the site of injury (Chen et al., 2015b). Collagen XV is abundantly found in the endoneurium and perineurium of adult peripheral nerves, and knockout mice display polyaxonal myelination, suggesting impairment in peripheral nerve maturation. Deficiency of collagen XV also leads to decreased nerve conduction velocities, implying involvement with myelination (Rasi et al., 2010).
The family of laminins also plays important roles in myelination and nerve repair. Laminin-211 is the dominant laminin isoform, a heterotrimeric molecule composed of laminin-α2, β1, and γ1 chains, and it is expressed in the basement membranes of both striated muscle and Schwann cells as well as forming the major constituent of the blood-brain barrier (Menezes et al., 2014; Durbeej, 2015). This heterotrimer self-assembles into a highly cross-linked network at cell surfaces, anchored by interactions with cell-surface receptors (Durbeej, 2015). In the PNS, the developing basal lamina is discontinuous, with axonal signals and the deposition of laminin-211 triggering the secretion of laminin-411, collagen IV, and heparin sulfate proteoglycans (HSPGs) and leading to polymerization of additional laminin-211 to create a dense basal lamina for myelinating SCs (Petersen et al., 2015). Mutations in the underlying gene, LAMA2, found on chromosome 6, are linked with a form of congenital muscular dystrophy, merosin-deficient CMD (MDC1A), which is characterized by alterations in peripheral nerves and skeletal muscle as well as white matter abnormalities and occasional mildly reduced peripheral nerve conduction velocities (Wewer and Engvall, 1996; Miyagoe-Suzuki et al., 2000; Durbeej, 2015). Gpr126 knockout models in mice result in radial sorting defects and impaired myelination, as do mutations in genes correlating with laminin-211 receptors (Petersen et al., 2015). Laminin-211 enhances the survival of newly formed OLs via interactions with integrin α6β1 (Colognato et al., 2002; Baron et al., 2005; Laursen et al., 2009) and regulates OPC proliferation through dystroglycan cleavage (Leiton et al., 2015).
Laminin-411 has been shown to be involved in axon segregation and myelination of peripheral nerves, with knockout mice displaying both motor and tactile sensory impairments (Wallquist et al., 2005; Rasi et al., 2010). Application of endogenous laminin has also been shown to induce regenerative changes in injury in both the peripheral and central nervous system (Politis, 1989).
Glycosaminoglycans (GAGs) are a family of linear polymers that make up a large component of the CNS ECM, with two members, hyaluronon and chondroitin sulfate proteoglycans, implicated in myelin regeneration (Wheeler and Fuss, 2016). Hyaluronon proteoglycans are known to accumulate in inflammatory lesions after spinal cord injury, with increased synthesis in demyelinated lesions of multiple sclerosis (MS) and the mouse model of MS (Back et al., 2005; Struve et al., 2005). This accumulation inhibits OPC differentiation and remyelination, as well as involvement in OPC maturation arrest in various white matter injury models (Buser et al., 2012; Bugiani et al., 2013). Degradation of hyaluronon by an OPC-specific hyaluoronidase named PH20 creates digestion products that cause inhibition of OPC maturation into myelinating mature OLs (Preston et al., 2013). These products likely interact with Toll-like receptor 2 (TLR2) found on OLs and upregulated in demyelinated lesions (Sloane et al., 2010), which leads to demyelination/failure of remyelination. Chondroitin sulfate proteoglycans (CSPG) are a major component of the inhibitory scar in MS lesions that suppresses axon regeneration (Pendleton et al., 2013). These lead to impaired OPC process outgrowth and remyelination through several methods, one of which is mechanical in nature – reduction of adhesion of OPCs and interference of the development of OPC processes (Lau et al., 2012). The use of a CSPG-degrading enzyme was sufficient to overcome this inhibition and treatment to reduce synthesis of CSPGs also improved overall remyelination capability (Lau et al., 2012). Additionally, CSPGs also interact with the protein tyrosine phosphatase sigma (PTPσ) receptor, whose downregulation reverses the inhibitory effects of CSPGs on remyelination (Pendleton et al., 2013).
Conclusions
aGPCRs are a dynamic group of signaling molecules that are responsible for a diverse set of developmental processes in the human body. They have an intimate relationship with the extracellular matrix, where many factors reside that not only work in combination with aGPCRs but also in complex with other signaling pathways. The process of myelination and nerve repair is multifactorial, and as new players emerge, their interplay becomes more intertwined. GPR126 is essential for the function of SCs in the PNS, and GPR56 is required for OPC proliferation in the CNS (Table 1). Each of these aGPCRs has multiple binding partners and likely has more that are yet to be discovered. Both SCs and OLs are regulated by a number of other factors, including ECM components such as collagens, laminins, and integrins, and oftentimes these molecules interact with each other. aGPCRs are attractive molecules to study, as they have significant potential as drug targets. At this time, the majority of aGPCRs are orphan receptors with undefined functions. As current knowledge about aGPCRs continues to increase, understanding of physiological functions associated with these receptors will also continue to deepen, allowing for therapeutic breakthroughs in the realm of human disease.
Table 1.
Characteristics of GPR56 and GPR126
| GPR56 | GPR126 | |
|---|---|---|
| Primary System of Action | CNS | PNS |
| Responsible Glial Cell | Oligodendrocytes | Schwann cells |
| Area of Regulation | Proliferation | Radial sorting and myelination |
| Endogneous Ligands | Collagen III (Transglutaminase 2) (in cancer) |
Laminin-211 Collagen IV PrPc |
| Downstream Signaling | RhoA | cAMP |
| Associated Human Disease | Bilateral Frontoparietal Polymicrogyria | Arthrogryposis Multiplex Congenita |
Acknowledgments
This work was supported by grants of National Institutes of Health, NS085201 and NS094164 to X.P) and T32HD007466 to P.M., and the National Multiple Sclerosis Society, RG-1501-02577 to X.P.
References
- Ackerman SD, Garcia C, Piao X, Gutmann DH, Monk KR. The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Galpha12/13 and RhoA. Nat Commun. 2015;6:6122. doi: 10.1038/ncomms7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, Luo NL, Banine F, Liu Y, Chang A, Trapp BD, Bebo BF, Jr, Rao MS, Sherman LS. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Baer AS, Syed YA, Kang SU, Mitteregger D, Vig R, Ffrench-Constant C, Franklin RJ, Altmann F, Lubec G, Kotter MR. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain. 2009;132:465–481. doi: 10.1093/brain/awn334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi-Buisson N, Poirier K, Boddaert N, Fallet-Bianco C, Specchio N, Bertini E, Caglayan O, Lascelles K, Elie C, Rambaud J, Baulac M, An I, Dias P, des Portes V, Moutard ML, Soufflet C, El Maleh M, Beldjord C, Villard L, Chelly J. GPR56-related bilateral frontoparietal polymicrogyria: further evidence for an overlap with the cobblestone complex. Brain. 2010;133:3194–3209. doi: 10.1093/brain/awq259. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, Ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Bremer J, Baumann F, Tiberi C, Wessig C, Fischer H, Schwarz P, Steele AD, Toyka KV, Nave KA, Weis J, Aguzzi A. Axonal prion protein is required for peripheral myelin maintenance. Nat Neurosci. 2010;13:310–318. doi: 10.1038/nn.2483. [DOI] [PubMed] [Google Scholar]
- Bugiani M, Postma N, Polder E, Dieleman N, Scheffer PG, Sim FJ, van der Knaap MS, Boor I. Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain. 2013;136:209–222. doi: 10.1093/brain/aws320. [DOI] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BS, Piao X, Bodell A, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, Voit T, Grant PE, Barkovich AJ, Walsh CA. Bilateral frontoparietal polymicrogyria: clinical and radiological features in 10 families with linkage to chromosome 16. Ann Neurol. 2003;53:596–606. doi: 10.1002/ana.10520. [DOI] [PubMed] [Google Scholar]
- Chen P, Cescon M, Bonaldo P. The Role of Collagens in Peripheral Nerve Myelination and Function. Mol Neurobiol. 2015a;52:216–225. doi: 10.1007/s12035-014-8862-y. [DOI] [PubMed] [Google Scholar]
- Chen P, Cescon M, Megighian A, Bonaldo P. Collagen VI regulates peripheral nerve myelination and function. FASEB J. 2014;28:1145–1156. doi: 10.1096/fj.13-239533. [DOI] [PubMed] [Google Scholar]
- Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, Vitale G, Feltri ML, Bonaldo P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 2015b;129:97–113. doi: 10.1007/s00401-014-1369-9. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Rothblum K, Stahl RC, Evans A, Prentiss L, Carey DJ. Glypican-1 and alpha4(V) collagen are required for Schwann cell myelination. J Neurosci. 2006;26:508–517. doi: 10.1523/JNEUROSCI.2544-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang NY, Chang GW, Huang YS, Peng YM, Hsiao CC, Kuo ML, Lin HH. Heparin interacts with the adhesion GPCR GPR56, reduces receptor shedding, and promotes cell adhesion and motility. J Cell Sci. 2016;129:2156–2169. doi: 10.1242/jcs.174458. [DOI] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, Ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- Detrait E, Laduron S, Meremans V, Baron-Van Evercooren A, van den Bosch de Aguilar P, Knoops B. Expression of integrins by murine MSC80 Schwann cell line: relationship to cell adhesion and migration. Neurosci Lett. 1999;267:49–52. doi: 10.1016/s0304-3940(99)00331-6. [DOI] [PubMed] [Google Scholar]
- Domingues HS, Portugal CC, Socodato R, Relvas JB. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front Cell Dev Biol. 2016;4:71. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbeej M. Laminin-alpha2 Chain-Deficient Congenital Muscular Dystrophy: Pathophysiology and Development of Treatment. Curr Top Membr. 2015;76:31–60. doi: 10.1016/bs.ctm.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol. 1987;105:1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27:247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Gallo V. The translational biology of remyelination: past, present, and future. Glia. 2014;62:1905–1915. doi: 10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Gloriam DE, Hoglund PJ, Lagerstrom MC, Schioth HB. There exist at least 30 human G-protein-coupled receptors with long Ser/Thr-rich N-termini. Biochem Biophys Res Commun. 2003a;301:725–734. doi: 10.1016/s0006-291x(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003b;554:381–388. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S, Deng Y, Luo R, Ackerman SD, Mogha A, Monk KR, Ying Y, Jeong SJ, Makinodan M, Bialas AR, Chang BS, Stevens B, Corfas G, Piao X. The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat Commun. 2015;6:6121. doi: 10.1038/ncomms7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TD, Talbot WS. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development. 2013;140:3167–3175. doi: 10.1242/dev.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martinez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210:153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann J, Aust G, Arac D, Engel FB, Formstone C, Fredriksson R, Hall RA, Harty BL, Kirchhoff C, Knapp B, Krishnan A, Liebscher I, Lin HH, Martinelli DC, Monk KR, Peeters MC, Piao X, Promel S, Schoneberg T, Schwartz TW, Singer K, Stacey M, Ushkaryov YA, Vallon M, Wolfrum U, Wright MW, Xu L, Langenhan T, Schioth HB. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev. 2015;67:338–367. doi: 10.1124/pr.114.009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Deng K, Siddiq MM, Pyie A, Mellado W, Hannila SS, Filbin MT. Cyclic AMP and Polyamines Overcome Inhibition by Myelin-Associated Glycoprotein through eIF5A-Mediated Increases in p35 Expression and Activation of Cdk5. J Neurosci. 2016;36:3079–3091. doi: 10.1523/JNEUROSCI.4012-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgert N. Novel human pathological mutations. Gene symbol: GPR98. Disease: Usher syndrome 2C. Hum Genet. 2009;125:342. [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SY, Shin YK, Park SY, Park JY, Lee HJ, Yoo YH, Kim JK, Park HT. Autophagic myelin destruction by Schwann cells during Wallerian degeneration and segmental demyelination. Glia. 2016;64:730–742. doi: 10.1002/glia.22957. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, Lloyd AC. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner CM, Long F, Solnica-Krezel L, Monk KR, Gray RS. Gpr126/Adgrg6 deletion in cartilage models idiopathic scoliosis and pectus excavatum in mice. Hum Mol Genet. 2015;24:4365–4373. doi: 10.1093/hmg/ddv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, Qiu X, Sharma S, Takimoto A, Ogura Y, Jiang H, Yan H, Kono K, Kawakami N, Uno K, Ito M, Minami S, Yanagida H, Taneichi H, Hosono N, Tsuji T, Suzuki T, Sudo H, Kotani T, Yonezawa I, Londono D, Gordon D, Herring JA, Watanabe K, Chiba K, Kamatani N, Jiang Q, Hiraki Y, Kubo M, Toyama Y, Tsunoda T, Wise CA, Qiu Y, Shukunami C, Matsumoto M, Ikegawa S. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45:676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- Kuffer A, Lakkaraju AK, Mogha A, Petersen SC, Airich K, Doucerain C, Marpakwar R, Bakirci P, Senatore A, Monnard A, Schiavi C, Nuvolone M, Grosshans B, Hornemann S, Bassilana F, Monk KR, Aguzzi A. The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature. 2016;536:464–468. doi: 10.1038/nature19312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenhan T, Aust G, Hamann J. Sticky signaling–adhesion class G protein-coupled receptors take the stage. Sci Signal. 2013;6:re3. doi: 10.1126/scisignal.2003825. [DOI] [PubMed] [Google Scholar]
- Langenhan T, Piao X, Monk KR. Adhesion G protein-coupled receptors in nervous system development and disease. Nat Rev Neurosci. 2016;17:550–561. doi: 10.1038/nrn.2016.86. [DOI] [PubMed] [Google Scholar]
- Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Doring A, Sloka S, Stirling DP, Rivest S, Yong VW. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol. 2012;72:419–432. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Chan CW, Ffrench-Constant C. An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci. 2009;29:9174–9185. doi: 10.1523/JNEUROSCI.5942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein PJ, Higgins D, Turner DC, Flier LA, Terranova VP. The NC1 domain of type IV collagen promotes axonal growth in sympathetic neurons through interaction with the alpha 1 beta 1 integrin. J Cell Biol. 1991;113:417–428. doi: 10.1083/jcb.113.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiton CV, Aranmolate A, Eyermann C, Menezes MJ, Escobar-Hoyos LF, Husain S, Winder SJ, Colognato H. Laminin promotes metalloproteinase-mediated dystroglycan processing to regulate oligodendrocyte progenitor cell proliferation. J Neurochem. 2015;135:522–538. doi: 10.1111/jnc.13241. [DOI] [PubMed] [Google Scholar]
- Li M, Shibata A, Li C, Braun PE, McKerracher L, Roder J, Kater SB, David S. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J Neurosci Res. 1996;46:404–414. doi: 10.1002/(SICI)1097-4547(19961115)46:4<404::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes RO, Walsh CA, Corfas G, Piao X. GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci. 2008;28:5817–5826. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher I, Schon J, Petersen SC, Fischer L, Auerbach N, Demberg LM, Mogha A, Coster M, Simon KU, Rothemund S, Monk KR, Schoneberg T. A tethered agonist within the ectodomain activates the adhesion G protein-coupled receptors GPR126 and GPR133. Cell Rep. 2014;9:2018–2026. doi: 10.1016/j.celrep.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Parker RM, Darby K, Eyre HJ, Copeland NG, Crawford J, Gilbert DJ, Sutherland GR, Jenkins NA, Herzog H. GPR56, a novel secretin-like human G-protein-coupled receptor gene. Genomics. 1999;55:296–305. doi: 10.1006/geno.1998.5644. [DOI] [PubMed] [Google Scholar]
- Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci U S A. 2011a;108:12925–12930. doi: 10.1073/pnas.1104821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Jin Z, Deng Y, Strokes N, Piao X. Disease-associated mutations prevent GPR56-collagen III interaction. PLoS One. 2012;7:e29818. doi: 10.1371/journal.pone.0029818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Yang HM, Jin Z, Halley DJ, Chang BS, MacPherson L, Brueton L, Piao X. A novel GPR56 mutation causes bilateral frontoparietal polymicrogyria. Pediatr Neurol. 2011b;45:49–53. doi: 10.1016/j.pediatrneurol.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Menezes MJ, McClenahan FK, Leiton CV, Aranmolate A, Shan X, Colognato H. The extracellular matrix protein laminin alpha2 regulates the maturation and function of the blood-brain barrier. J Neurosci. 2014;34:15260–15280. doi: 10.1523/JNEUROSCI.3678-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, Ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagoe-Suzuki Y, Nakagawa M, Takeda S. Merosin and congenital muscular dystrophy. Microsc Res Tech. 2000;48:181–191. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<181::AID-JEMT6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33:17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Feltri ML, Taveggia C. New insights on Schwann cell development. Glia. 2015;63:1376–1393. doi: 10.1002/glia.22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Oshima K, Jors S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138:2673–2680. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moteki H, Yoshimura H, Azaiez H, Booth KT, Shearer AE, Sloan CM, Kolbe DL, Murata T, Smith RJ, Usami S. USH2 caused by GPR98 mutation diagnosed by massively parallel sequencing in advance of the occurrence of visual symptoms. Ann Otol Rhinol Laryngol. 2015;124(Suppl 1):123S–128S. doi: 10.1177/0003489415574070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave KA, Werner HB. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- Nicholas RS, Wing MG, Compston A. Nonactivated microglia promote oligodendrocyte precursor survival and maturation through the transcription factor NF-kappa B. Eur J Neurosci. 2001;13:959–967. doi: 10.1046/j.0953-816x.2001.01470.x. [DOI] [PubMed] [Google Scholar]
- Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci Signal. 2014;7:ra76. doi: 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini LA, Millet V, Hoyos HC, Giannoni JP, Croci DO, Marder M, Liu FT, Rabinovich GA, Pasquini JM. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011;18:1746–1756. doi: 10.1038/cdd.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML, McDonald JW. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPsigma. Exp Neurol. 2013;247:113–121. doi: 10.1016/j.expneurol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, Ghidinelli M, Feltri ML, Schoneberg T, Piao X, Monk KR. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron. 2015;85:755–769. doi: 10.1016/j.neuron.2014.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao X, Chang BS, Bodell A, Woods K, Benzeev B, Topcu M, Guerrini R, Goldberg-Stern H, Sztriha L, Dobyns WB, Barkovich AJ, Walsh CA. Genotype-phenotype analysis of human frontoparietal polymicrogyria syndromes. Ann Neurol. 2005;58:680–687. doi: 10.1002/ana.20616. [DOI] [PubMed] [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, Voit T, Ross ME, Michaud JL, Descarie JC, Barkovich AJ, Walsh CA. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- Politis MJ. Exogenous laminin induces regenerative changes in traumatized sciatic and optic nerve. Plast Reconstr Surg. 1989;83:228–235. doi: 10.1097/00006534-198902000-00004. [DOI] [PubMed] [Google Scholar]
- Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Back SA, Sherman LS. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–280. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci. 2012;15:1074–1077. doi: 10.1038/nn.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- Rasi K, Hurskainen M, Kallio M, Staven S, Sormunen R, Heape AM, Avila RL, Kirschner D, Muona A, Tolonen U, Tanila H, Huhtala P, Soininen R, Pihlajaniemi T. Lack of collagen XV impairs peripheral nerve maturation and, when combined with laminin-411 deficiency, leads to basement membrane abnormalities and sensorimotor dysfunction. J Neurosci. 2010;30:14490–14501. doi: 10.1523/JNEUROSCI.2644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U. Brain matrix: structure, turnover and necessity. Biochem Soc Trans. 2007;35:656–660. doi: 10.1042/BST0350656. [DOI] [PubMed] [Google Scholar]
- Ravenscroft G, Nolent F, Rajagopalan S, Meireles AM, Paavola KJ, Gaillard D, Alanio E, Buckland M, Arbuckle S, Krivanek M, Maluenda J, Pannell S, Gooding R, Ong RW, Allcock RJ, Carvalho ED, Carvalho MD, Kok F, Talbot WS, Melki J, Laing NG. Mutations of GPR126 are responsible for severe arthrogryposis multiplex congenita. Am J Hum Genet. 2015;96:955–961. doi: 10.1016/j.ajhg.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roda RH, FitzGibbon EJ, Boucekkine H, Schindler AB, Blackstone C. Neurologic syndrome associated with homozygous mutation at MAG sialic acid binding site. Ann Clin Transl Neurol. 2016;3:650–654. doi: 10.1002/acn3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman GS, Ackerman SD, Ding C, Koide A, Leon K, Luo R, Stoveken HM, Fernandez CG, Tall GG, Piao X, Monk KR, Koide S, Arac D. Structural Basis for Regulation of GPR56/ADGRG1 by Its Alternatively Spliced Extracellular Domains. Neuron. 2016;91:1292–1304. doi: 10.1016/j.neuron.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J, Grund EM, Silva HM, Lafaille JJ, Fishell G, Salzer JL. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature. 2015;526:448–452. doi: 10.1038/nature14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34:2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Lin ST, Fu YH, Ptacek LJ. Very large G protein-coupled receptor 1 regulates myelin-associated glycoprotein via Galphas/Galphaq-mediated protein kinases A/C. Proc Natl Acad Sci U S A. 2013;110:19101–19106. doi: 10.1073/pnas.1318501110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A. 2010;107:11555–11560. doi: 10.1073/pnas.1006496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoveken HM, Hajduczok AG, Xu L, Tall GG. Adhesion G protein-coupled receptors are activated by exposure of a cryptic tethered agonist. Proc Natl Acad Sci U S A. 2015;112:6194–6199. doi: 10.1073/pnas.1421785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve J, Maher PC, Li YQ, Kinney S, Fehlings MG, Kuntz Ct, Sherman LS. Disruption of the hyaluronan-based extracellular matrix in spinal cord promotes astrocyte proliferation. Glia. 2005;52:16–24. doi: 10.1002/glia.20215. [DOI] [PubMed] [Google Scholar]
- Van Strien ME, Baron W, Bakker EN, Bauer J, Bol JG, Breve JJ, Binnekade R, Van Der Laarse WJ, Drukarch B, Van Dam AM. Tissue transglutaminase activity is involved in the differentiation of oligodendrocyte precursor cells into myelin-forming oligodendrocytes during CNS remyelination. Glia. 2011;59:1622–1634. doi: 10.1002/glia.21204. [DOI] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lannergren J, Domogatskaya A, Ogren SO, Risling M, Hammarberg H, Tryggvason K, Cullheim S. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J Neurosci. 2005;25:3692–3700. doi: 10.1523/JNEUROSCI.5225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergard L, Christensen HM, Harris DA. The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta. 2007;1772:629–644. doi: 10.1016/j.bbadis.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MD, Luijendijk MW, Humphrey KD, Moller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet. 2004;74:357–366. doi: 10.1086/381685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer UM, Engvall E. Merosin/laminin-2 and muscular dystrophy. Neuromuscul Disord. 1996;6:409–418. doi: 10.1016/s0960-8966(96)00384-7. [DOI] [PubMed] [Google Scholar]
- Wheeler NA, Fuss B. Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp Neurol. 2016;283:512–530. doi: 10.1016/j.expneurol.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP. Association of GPR126 gene polymorphism with adolescent idiopathic scoliosis in Chinese populations. Genomics. 2015;105:101–107. doi: 10.1016/j.ygeno.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Xu L, Begum S, Hearn JD, Hynes RO. GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci U S A. 2006;103:9023–9028. doi: 10.1073/pnas.0602681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang XF, Tong Y, Jin ZB. Targeted exome sequencing identified two novel truncation mutations in GPR98 causing Usher syndrome. Clin Exp Ophthalmol. 2016;44:197–199. doi: 10.1111/ceo.12664. [DOI] [PubMed] [Google Scholar]
- Yang L, Chen G, Mohanty S, Scott G, Fazal F, Rahman A, Begum S, Hynes RO, Xu L. GPR56 Regulates VEGF production and angiogenesis during melanoma progression. Cancer Res. 2011;71:5558–5568. doi: 10.1158/0008-5472.CAN-10-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisen J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Zarinfard G, Tadjalli M, Razavi S, Kazemi M. Effect of Laminin on Neurotrophic Factors Expression in Schwann-Like Cells Induced from Human Adipose-Derived Stem Cells In Vitro. J Mol Neurosci. 2016 doi: 10.1007/s12031-016-0808-6. [DOI] [PubMed] [Google Scholar]
- Zendman AJ, Cornelissen IM, Weidle UH, Ruiter DJ, van Muijen GN. TM7XN1, a novel human EGF-TM7-like cDNA, detected with mRNA differential display using human melanoma cell lines with different metastatic potential. FEBS Lett. 1999;446:292–298. doi: 10.1016/s0014-5793(99)00230-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


