Fig. 2.
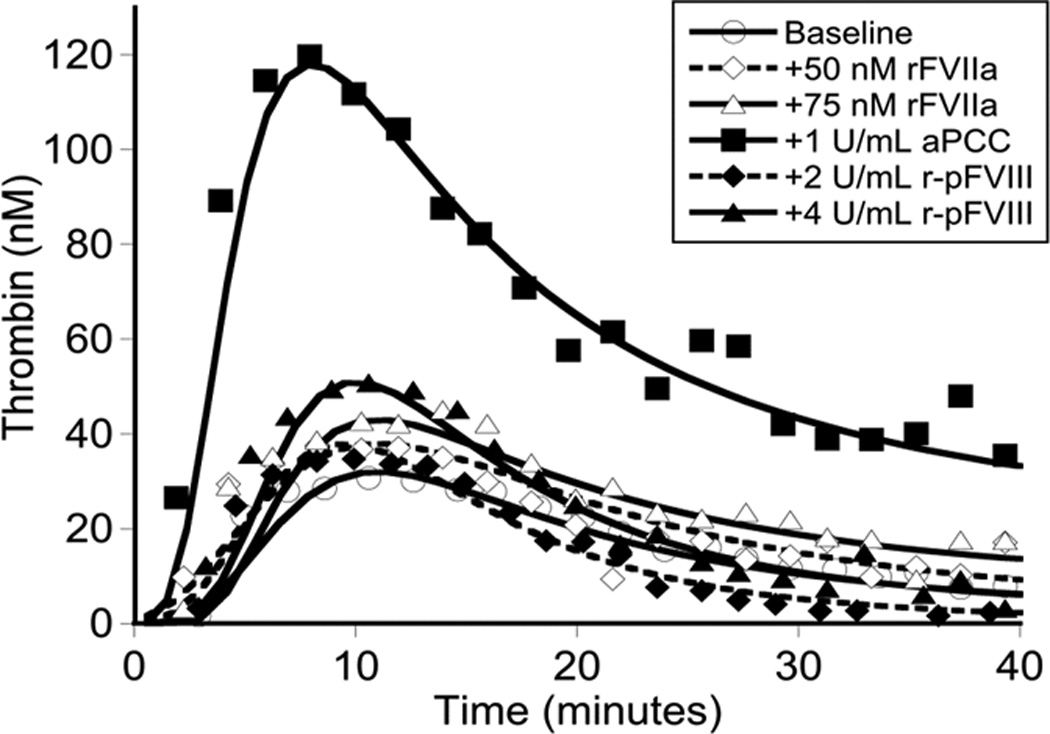
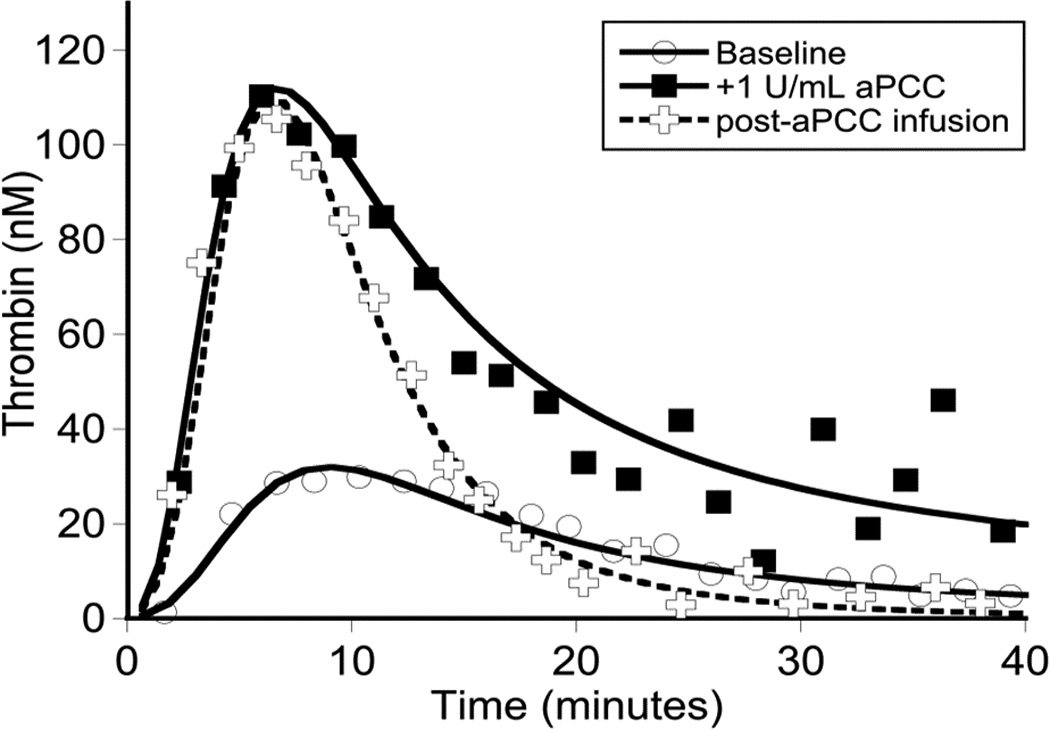
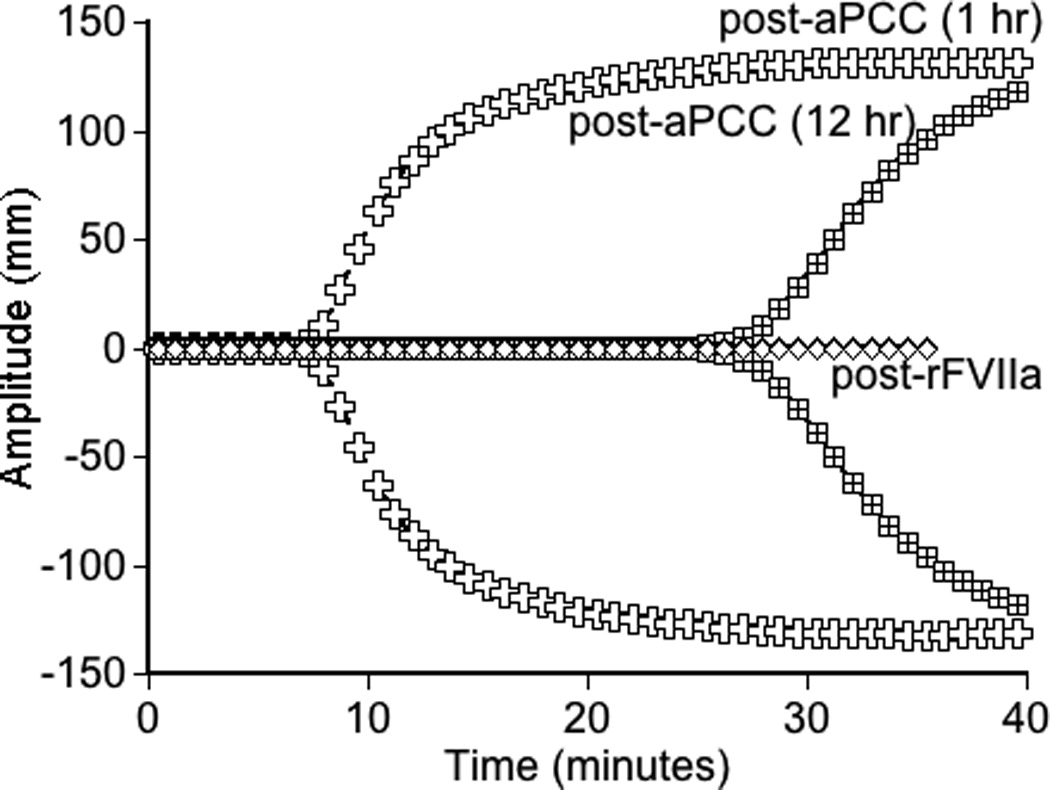
Global assays of the patient’s hemostatic potential at baseline and in response to rFVIIa, aPCC, and r-pFVIII were evaluated. At time of plasma sampling the patient’s hFVIII inhibitor titer was 37 BU and pFVIII inhibitor titer was 3 BU. A. Thrombin generation of the patient’s platelet poor plasma at baseline and following in vitro spiking with hemostatic agents. The final concentration of rFVIIa was chosen to reproduce a clinical dose of 180 µg/kg or 270 µg/kg. The final concentration of aPCC was chosen to reproduce a clinical dose of 100 IU/kg. The final concentration of r-pFVIII was chosen to reproduce a clinical dose of 100 IU/kg or 200 IU/kg. B. Thrombin generation examined in the patient’s platelet poor plasma at baseline and after ex vivo spiking with 1 IU/ml aPCC compared to the patient’s platelet poor plasma sampled after in vivo infusion of 100 IU/kg of aPCC to provide hemostatic coverage for CVL removal/replacement. C. TEG assayed in whole blood collected 1 hour and 12 hours following pre-operative infusion of 100 IU/kg of aPCC, at the same time as plasma was assayed in panel B. Also assayed in the clinical coagulation laboratory was whole blood collected at 15 minutes following in vivo infusion of 270 µg/kg of rFVIIa, demonstrating a TEG tracing that did not differ from the patient’s baseline.
