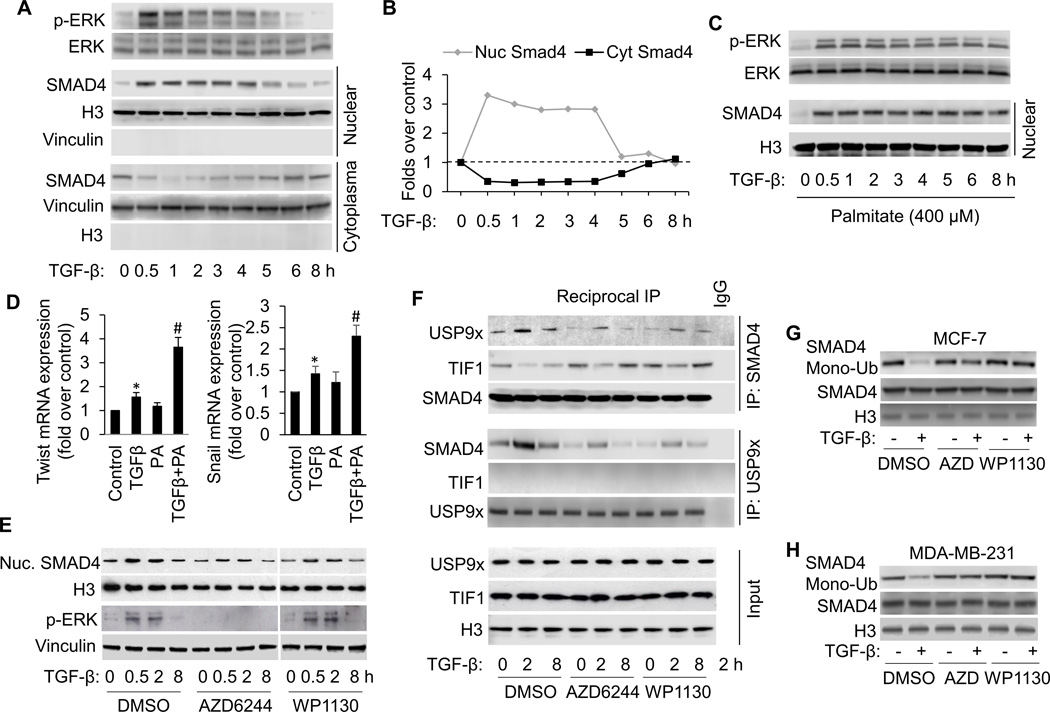
Figure 1.
FFA facilitates TGF-β-induced ERK activation, which promotes TGFβ-induced SMAD4 nuclear localization and gene expression by USP9x-SMAD4 interaction and nuclear SMAD4 deubiquitination. A, Western blot analysis of p-ERK and nuclear SMAD4 in MCF-7 cells treated with 3 ng/ml of TGF-β according to the indicated time points. B, The Western blots were quantitated, and the results are presented graphically to show the dynamics of SMAD4 nuclear cytoplasmic shuttling. C, Western blot analysis of p-ERK and nuclear SMAD4 in MCF-7 cells treated with 3 ng/ml of TGF-β in the presence of palmitate (PA, 400 µM) for the shown time periods. D, Induction of Twist and Snail mRNA expression in MCF-7 cells treated with TGFβ1 in the presence or absence of PA for 8 hours analyzed by qRT-PCR. All mRNA are normalized to PUM1 and presented as fold (mean ± SD) over Control based on three experiments. *p<0.05 versus Control; #p < 0.05 versus TGFβ. E, Western blot analysis of p-ERK and nuclear SMAD4 in MCF-7 cells treated with 3 ng/ml of TGF-β in the presence or absence of AZD6244 (1 µM) or WP1130 (5 µM). To detect nuclear USP9x-SMAD4 interaction, TIF1γ-SMAD4 interaction (F) and SMAD4 modifications (G and H), cells were also treated with 15 ng/ml of LMB to block SMAD4 nuclear export. F, Reciprocal co-immunoprecipitations (co-IP) were performed with lysates from MCF-7 cells using anti-SMAD4 and anti-USP9x antibodies and Western blot analysis was performed to detect SMAD4, USP9x and TIF1γ, respectively in IP elutes and in whole cell lysates (input). G and H, Nuclear SMAD4 ubiquitination was detected by transfecting cells with WT-SMAD4 and HA-ubiquitin, immunoprecipitation with anti-Smad4 antibody, followed by immunoblotting with anti-HA antibody.