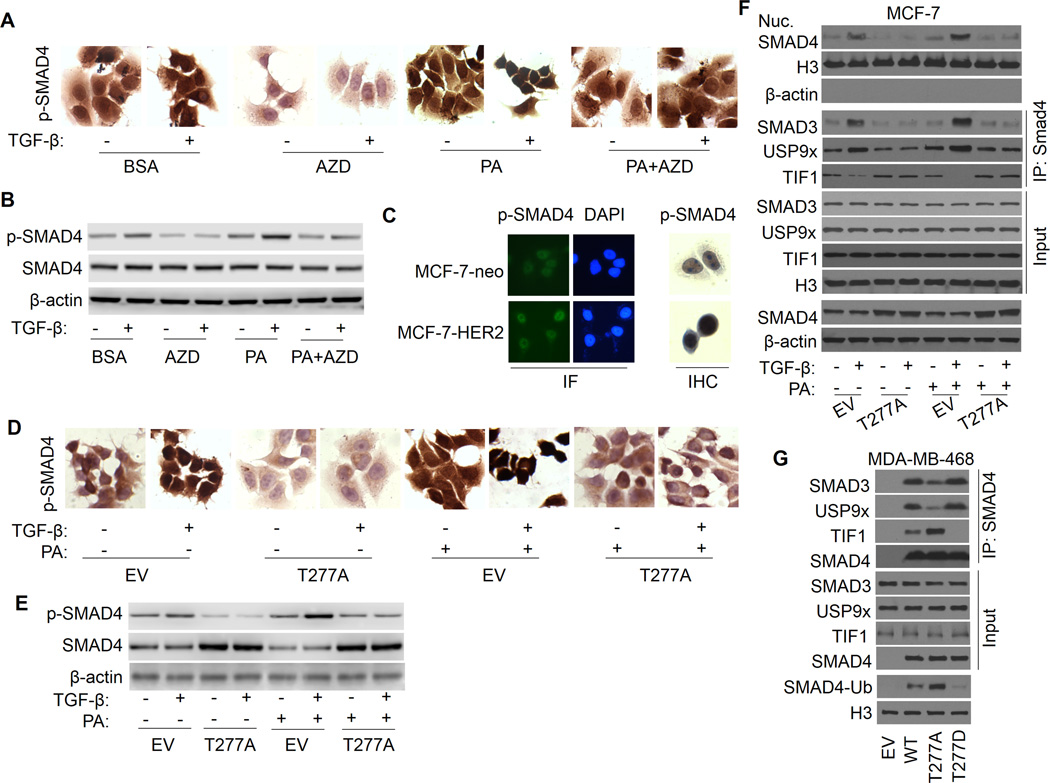
Figure 4.
SMAD4 T277 phosphorylation is responsible for FFA promotion of TGF-β-induced USP9x-SMAD4 interaction, nuclear SAMD4 retention, SMAD3-SMAD4 complex formation. A, MCF-7 cells were treated with AZD6244, PA or PA plus AZD6244 in the presence or absence of TGF-β for 2 h and stained with IHC method using anti-phospho-SMAD4 (Thr277) antibody. B, Whole-cell extracts were prepared and subjected to Western analysis using anti-phospho-SMAD4, SMAD4 and actin antibodies. C, MCF-7-neo and MCF-7-HER2 cells were stained with IF and IHC method using anti-phospho-SMAD4 antibody. D, MCF-7 cells transfected with SMAD4-T277A or empty vector control (EV) were treated with BSA or PA in the presence or absence of TGF-β1 and stained with IHC method for anti-phospho-SMAD4 antibody. E, Whole-cell extracts were prepared and subjected to Western analysis using anti-phospho-SMAD4, SMAD4 and actin antibodies. F, MCF-7 cells were transfected with EV or SMAD4-T277A, and nuclear extract was prepared. SMAD4-SMAD3, SMAD4-USP9x, and SMAD4-TIF1γ interaction were determined by immunoprecipitation with SMAD4 antibody, followed by immunoblotting with SMAD3, USP9x, or TIF1γ antibody. G, MDA-MB-468 cells were transfected with EV, WT-SMAD4, SMAD4-T277A, or SMAD4-T277D, and nuclear SMAD4-SMAD3, SMAD4-USP9x, and SMAD4-TIF1γ interaction were determined by immunoprecipitation and immunoblotting as described above. SMAD4 monoubiquitination was also detected.