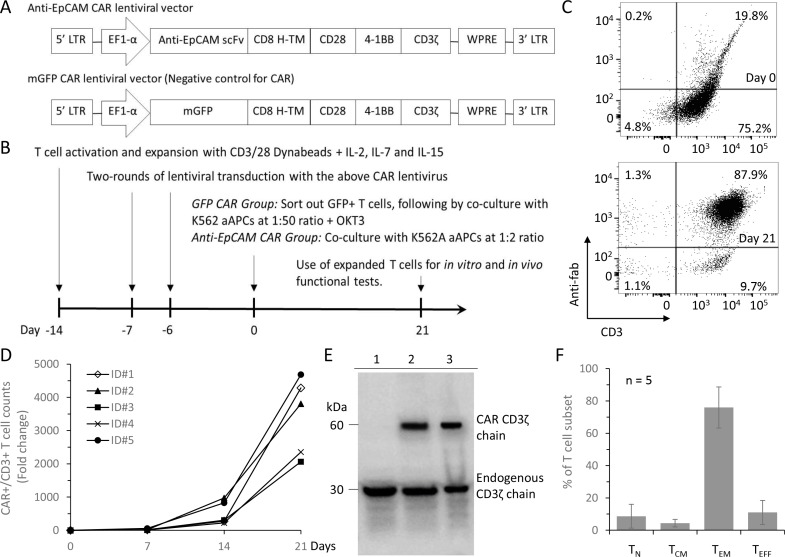
Figure 2. Generation and expansion of EpCAM-specific CART cells.
(A) Schematics of lentiviral vectors used in the study. The scFv region that recognizes EpCAM was derived from 4D5MOC-B humanized mAb. Anti-EpCAM CAR contains the CD28 and 4-1BB co-stimulatory domains and the CD3zeta T cell activation domain. mGFP CAR control vector was constructed using the GFP sequence instead of EpCAM-specific scFv. (B) Schematic illustration of EpCAM-specific CART cell generation and expansion. (C) Flow cytometric analysis to detect the surface expression of anti-EpCAM CAR on the genetically modified T cells before and after 21 days of co-culturing with K562A-EpCAM aAPCs using anti-mouse IgG Fab and anti-human CD3 antibodies. Representative FACS plots are shown. (D) Increase in the number of CD3+CAR+ T cells over the co-culturing with K562A-EpCAM cells. The number of initially seeded T cells was 2E4 per well. Results from five different PBMC samples are shown. (E) Western blot analysis using a CD3ζ-specific antibody confirms the CAR expression in modified T cells 21 days after co-culture with K562A-EpCAM cells. The endogenous CD3ζ was stained as an internal loading control. Lane 1: unmodified T cells; Lane 2: T cells transduced with mGFP CAR lentiviral vectors; Lane 3: T cells transduced with anti-EpCAM CAR lentiviral vectors. (F) Phenotyping of the propagated EpCAM-specific CART cells. T cells collected after 21 days of co-culture with K562A-EpCAM cells were assessed by flow cytometry. T cells were gated for the presence of naive (TN, CCR7+CD45RA+), central memory (TCM, CCR7+CD45RA-), effector memory (TEM, CCR7-CD45RA-), and terminally differentiated effector T cells (TEFF, CCR7-CD45RA+). Results from five different PBMC samples are shown.