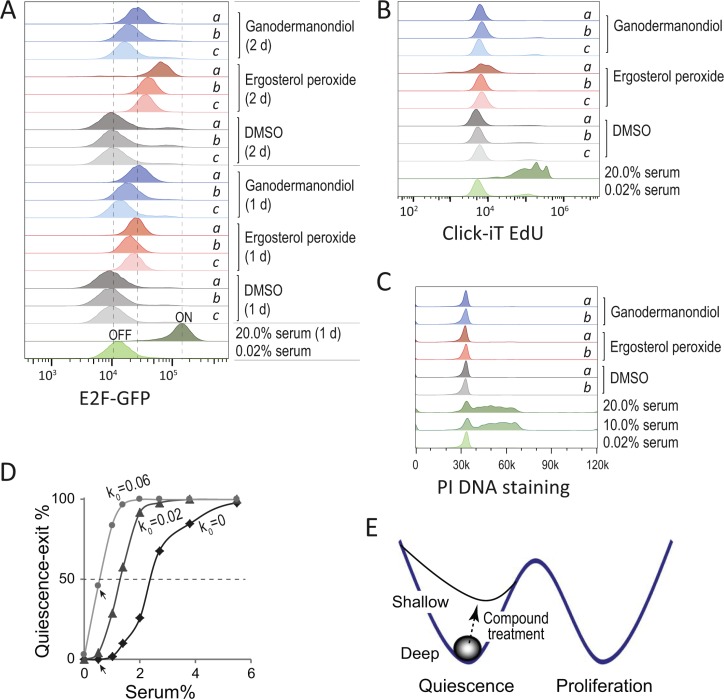
Figure 3. Compound treatment reduces quiescence depth.
(A–C) Treated cells remained quiescent but at a shallower state with increased E2F expression. REF/E23 cells were induced to quiescence by serum starvation (0.02% serum) for 2 days, and subsequently treated with ergosterol peroxide, ganodermanondiol, DMSO control, or serum stimulation (10%, 20% serum) controls as indicated. Treatment doses of a, b, c corresponded to 20, 10, and 5 μg/ml, respectively. The E2F-GFP reporter activities (A) were measured after 1 and 2 day(s) of treatment, with the E2F-OFF and -ON levels labelled alongside the serum starved (0.02%) and stimulated (20%) control samples, respectively. The Click-iT EdU assays (B) were performed after 1.5 days of treatment to detect subpopulations of cells that exited quiescence (with positive EdU incorporation) and divided (with incorporated EdU intensity reduced by half). The PI DNA staining assays (C) were performed after 1 day of treatment for cell cycle profile analysis. (D) Computer simulated percentages of cells that exit quiescence (y-axis) in response to serum input at indicated concentrations (x-axis). Quiescence exit was determined by the event of turning ON the Rb-E2F bistable switch in stochastic model simulations (see Methods) [21]. The serum concentration corresponding to 50% quiescence exit indicated the activation threshold of the Rb-E2F bistable switch. The parameter k0 corresponded to the rate constant of basal E2F expression. Arrow-pointed data points refer to percentages of quiescence-exit cells with k0 =0 and 0.06, respectively, at the 0.5% low serum condition. (E) Shallow vs. deep quiescence in compound-treated cells. Ergosterol peroxide and ganodermanondiol reduce the quiescence depth of treated cells, which are primed for quiescence exit and cell cycle re-entry compared to non-treated cells.