Figure 1. Genetic changes present in t-MN were not detectable at primary cancer diagnosis.
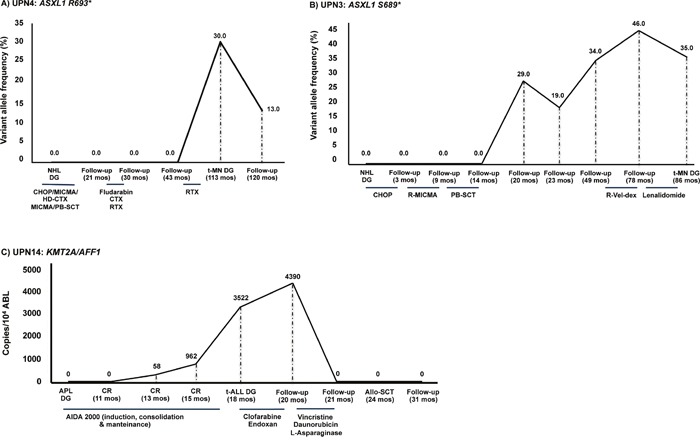
A. In UPN4, the ASXL1 R693* mutation detected in the t-MN sample was undetectable at the time of NHL diagnosis (113 months prior to t-MN onset). B. In UPN3, the ASXL1 S689* mutation was undetectable at the time of primary cancer diagnosis (NHL) but was detectable for the first time during NHL follow-up (at 20 months) and increased until the time of t-MN diagnosis (at 86 months). Of note, the mutation was first detected after high-dose therapy and PB-SCT. C. Similarly, the KMT2A-AFF1 fusion identified in UPN 14 at t-ALL diagnosis was, as expected, undetectable in the primary APL diagnostic sample, and was detected at low levels by Q-RT-PCR thirteen months after achievement of complete molecular remission of APL, using the AIDA 2000 protocol [17]. Notably, a constant increase in the transcript copy number was evident from first identification (58 copies/104 ABL) to t-ALL onset (3522 copies/104 ABL). The fusion transcript became undetectable only after re-induction treatment according to the LAL0904 protocol [27] and resulted to date undetectable (54+ months) after ASCT. The variant allele frequency (VAF) is indicated. Legend. NHL DG: non-Hodgkin lymphoma diagnosis; t-MN DG: therapy-related myeloid neoplasm diagnosis; APL DG: acute promyelocitic leukemia diagnosis; CR: complete remission; t-ALL DG: therapy-related acute lymphoblastic leukemia diagnosis; Allo-SCT: allogeneic stem cell transplantation; CHOP: cyclophosphamide, adriblastin, vincristine, prednisone; MICMA: mitoxantrone, carboplatin, cytarabine, methylprednisolone; PB-SCT: peripheral blood stem cell transplantation; RTX: radiotherapy; CTX: cyclophosphamide; HD-CTX: high-dose cyclophosphamide; R-MICMA: mitoxantrone, carboplatin, cytosine arabinoside, and methylprednisolone; R-Vel-dex: lenalidomide, bortezomib, dexametasone; AIDA: ATRA, idarubicine.
