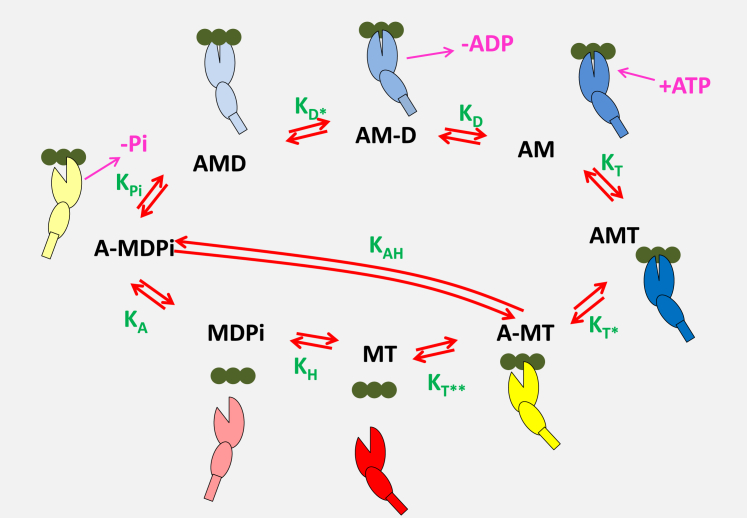
Figure 1.
A minimal ATPase actin-myosin cycle is defined by eight states and nine equilibrium constants. The actin monomers, A, along an actin filament are shown as three green solid circles, and the motor domain of myosin, M, is represented as two ellipses, the larger representing the upper and lower 50 k domain, with a cleft separating the two domains, and the smaller representing the converter domain, with a rectangular lever arm. The relative movements of these domains represent the structural conformations of myosin in each state. The actin-myosin cycle includes ATP binding to rigor-like complex, AM, forming the AMT state (equilibrium constant for the transition, = [AMT]/[AM][T]), followed by a rapid change in myosin conformation leading to the cleft opening in the A-MT state and subsequent rapid detachment of actin . A conformational change in the head following the opening of the 50 kDa cleft leads to the recovery stroke and hydrolysis of ATP transitioning into the MDPi state . Then, the myosin can reattach to actin, forming the A-MDPi state and after the cleft closure, the power stroke and Pi release, transitioning into an AMD state in which ADP remains firmly attached to myosin. This state allows a conformational change into the AM-D state , from which ADP is released and the cycle is completed. The step labeled allows for possible hydrolysis of ATP while myosin remains attached to actin. For simplicity, ATP and ADP in state labeling are denoted as T and D, respectively, and inorganic phosphate as Pi. A hyphen indicates a relatively weak association between the species. The equilibrium constants between the states are denoted in green letters and represent a ratio between forward (clockwise) and reverse state-transition rate constants. Note that the nomenclature for rate and equilibrium constants uses the subscripts A, D, Pi, and T to indicate the species binding to or released from myosin in each second-order step. An asterisk (∗) indicates that an isomerization event is linked with the second-order binding or dissociation event. The only exceptions are and , which are the ATP hydrolysis steps with or without actin attached.