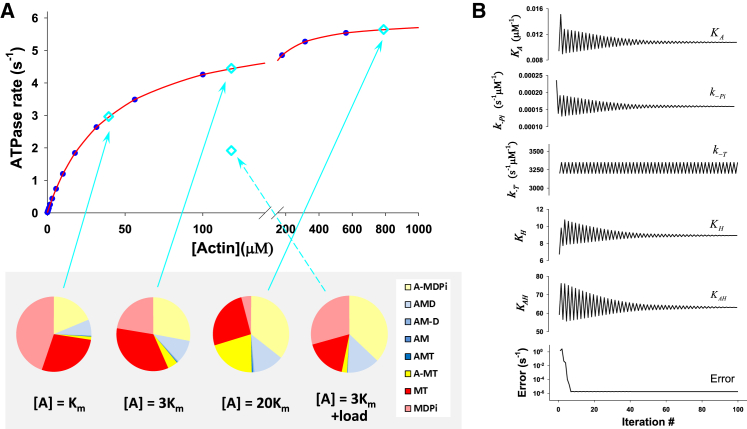
Figure 2.
Modeling the ATPase cycle for β-cardiac myosin. (A) The best fit of ATPase rate versus actin concentration, [A], for HC WT β-S1. Data in blue are the predicted ATPase rates based on the published and values, and data in red indicate the best fit of the model in Fig. 1 to the data. The constants used and derived in the fitting are listed in Table 1. The state occupancies for each of the intermediates in the cycle are given in a pie chart, color coded to match the intermediates in Fig. 1. These are shown for three actin concentrations (blue diamonds), [A] = , 3, and 20, and at 3 under load (see text). (B) The convergence of the equilibrium constants, , , and , and reverse rate constants, , and, , by DLS for the damping parameter ε = 0.25 (21). All free parameters converge at 30–50 iterations except , where value varies between 3200 and 3350 s−1, but this variation does not affect the error, suggesting that the value of this parameter cannot be resolved for this experiment (see Table 1). The mean-square error (21) converges quickly to a normalized value, Error = <2 × 10−6 s−1, and the fit of data is excellent. The resolution matrix for this fitting procedure is listed in Table 2.