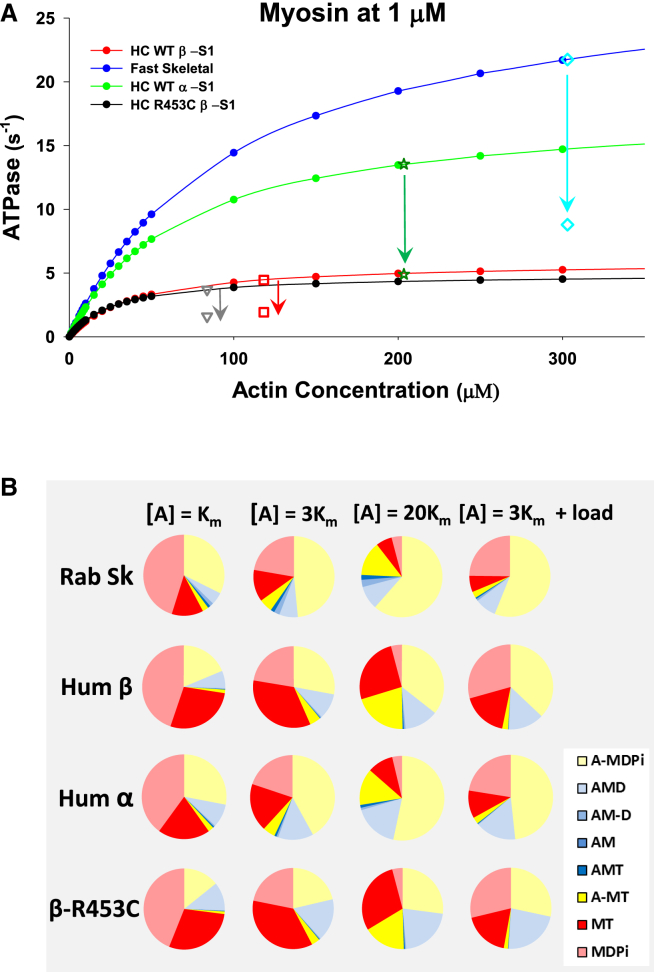
Figure 3.
Details of the fit to the ATPase data and the state occupancy of intermediates in the ATPase cycle for four muscle myosin isoforms. (A) The dependence of the ATPase cycle on actin concentration and (B) the occupancy of each of the intermediates in the ATPase cycle at three different actin concentrations, [A] = , 3, and 20 , corresponding to 0.5, 0.75, and 0.95 of . The presentation also includes predictions for how the state occupancy would change for the [A] = 3 conditions if the actomyosin system were loaded such that the isomerization steps controlling both Pi and ADP release were reduced by a factor of 3 (see text for details). The values generated for the pie charts are given in Table S2. Note that low-actin-concentration-state occupancies were dominated by MDPi (range 0.40–0.45) and to a lesser extent by A-MDPi (0.19–0.32). The MT form was more variable (0.13–0.29), reflecting the wide variation in the rate constant of the ATP hydrolysis step . As actin increased, there was a shift in occupancy from MDPi to A-MDPi, as expected with MDPi almost negligible at [A] =20. A-MDPi dominates (0.35–0.62) at [A] = 20, but with significant contributions from both MT (0.06–0.26) and A-MT (0.14–0.21). The presence of a large amount of A-MT is due to the value of the weak affinity of MT for actin, here assigned to 1 mM at 25 mM KCl and expected to be three times weaker at 100 mM KCl. Thus, the A-MT would only be significant in a sarcomere if the local actin concentration exceeded 1 mM at physiological salt concentrations. The occupancy of MT is much larger in β- than in α- and skeletal isforms, reflecting the slower hydrolysis rate constant for β. The major form tightly bound to actin was AMD, and this had a higher occupancy in β and α than in skeletal myosin at all actin concentrations, reflecting the slower ADP release rate constants in the β- and α-isoforms.