Abstract
Tetraarylmethane derivatives are desirable for a variety of applications, but difficult to access with modern C–C bond-forming reactions. Here we report a straightforward method for palladium-catalysed arylation of aryl(heteroaryl)methanes and diaryl(heteroaryl)methanes with aryl chlorides. This reaction enables introduction of various aryl groups to construct triaryl(heteroaryl)methanes via a C–H functionalization in good to excellent yield, and represents the first step towards a general transition metal catalysed synthesis of tetraarylmethanes.
Molecules with 4 aryl groups attached to a central carbon atom have proven challenging to synthesize despite their attractiveness for a variety of applications. Here the authors report a Pd-catalysed arylation of di- and triarylmethanes, giving a high-yielding route to an array of tetraarylmethanes.
Tetraarylmethanes and related derivatives are important building blocks, with uses ranging from molecular devices1,2,3,4 to porous organic frameworks5,6,7 and applications from protein translocation detection8 to drug delivery9. Furthermore, a recent study of 9,000 bioactive compounds by AstraZeneca to evaluate molecular space concluded that most biologically active compounds are linear or disk shaped, and very few are sphere-like molecules. They concluded that chemists should ‘… expand the current arsenal of tools to access less populated space …' and ‘… this may prove advantageous as the pharmaceutical industry ventures into new disease areas and new target classes which require different molecular shapes to bind and achieve the desired effect'10. Tetraarylmethanes are members of sphere-like molecules that have not been widely explored due to synthetic difficulties.
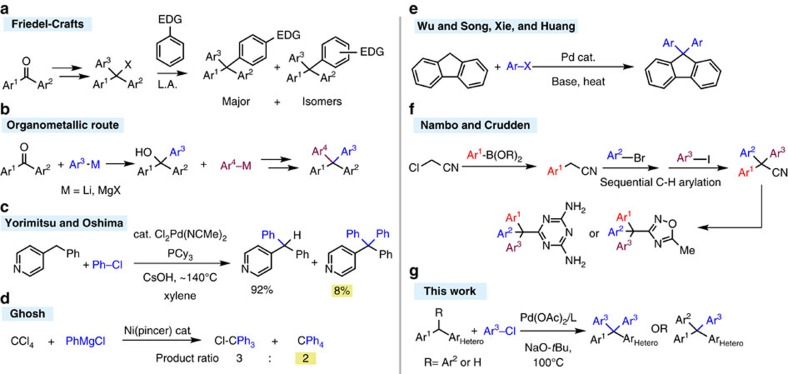
The classic methods to synthesize tetraarylmethanes are based on Friedel-Crafts arylations (Fig. 1a)11,12,13,14,15,16,17,18 and nucleophilic addition of alkyllithium or Grignard reagents to benzophenone derivatives (Fig. 1b)19,20,21. Each of these approaches has well-known limitations. Friedel-Crafts reactions require arenes with electron-donating groups, often afford mixtures of regioisomers, and are not suitable when meta-substituted products are required. Aryl organometallic reagents are typically highly reactive, sensitive to traces of air and moisture, and exhibit limited functional group tolerance.
Figure 1. Synthesis of tetraarylmethanes.
(a,b) Classic approaches to tetraarylmethanes include the Friedel-Crafts electrophilic aromatic substitution and organometallic additions to triarylmethyl cation precursors. (c) Formation of 8% tetraarylmethane byproduct in the synthesis of triarylmethanes. (d) Arylation of carbon tetrachloride resulted in up to 39% conversion to tetraphenylmethane. (e) Arylation of fluorene. (f) Sequential arylation/cycloaddition. (g) This work: arylation of 4-benzyl pyridine and (heteroaryl)diphenylmethanes to yield tetraarylmethane derivatives.
Transition metal-catalysed cross-coupling reactions have emerged as an excellent method to construct C–C bonds. For example, they have been used with great success in the coupling of aryl halides to diarylmethanes to afford triarylmethanes22,23,24,25 (Fig. 1c)26. The application of transition metal catalysts to the synthesis of tetraarylmethanes, however, has proven quite challenging. This may be due to difficulties associated with the transmetallation of the bulky triarylmethyl organometallic species. Thus, there is tremendous unrealized potential in the transition metal catalysed construction of tetraarylmethane derivatives.
We are only aware of a handful of metal catalysed syntheses of tetraarylmethane derivatives. In their synthesis of triarylmethanes, Yorimitsu, Oshima and co-workers noted formation of 8% yield of a tetraarylmethane (Fig. 1c). Despite the intriguing nature of this byproduct, subsequent reports have not been forthcoming. Ghosh and co-workers reported the nickel-catalysed reaction of carbon tetrachloride and PhMgCl to form a 3:2 ratio of triphenylmethyl chloride to tetraphenylmethane (Fig. 1d)27. These products were not isolated. Recently, the palladium-catalysed arylation of fluorenes by the teams of Wu and Song, Xie, and Huang was reported to give diarylfluorenes (Fig. 1e)28,29. Fluorene is more acidic and less sterically demanding than other diarylmethane derivatives. In significant work, the team of Nambo and Crudden outlined the generation of triarylacetonitriles, Ar3C–CN, then assemble a 4th heteroaryl group by building on the nitrile (Fig. 1f)30.
Herein, we present a novel palladium-catalysed method for the synthesis of heteroaryl-substituted tetraarylmethane derivatives (Fig. 1g). This effort represents the first high-yielding transition metal catalysed preparation of tetraarylmethane derivatives. It enables the synthesis of a broad range of triaryl(heteroaryl)methanes, including those with four different aryl groups attached to the central carbon atom.
Results
Preliminary catalyst screening
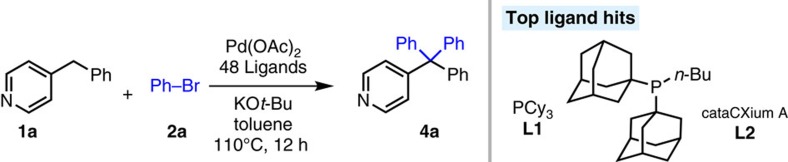
Given that the only example of arylation of a triarylmethane derivative we are aware of is Yorimitsu, Oshima and co-workers' 8% yield of triphenyl(4-pyridyl)methane (Fig. 1c), we chose this starting point for our reaction design. Instead of CsOH, we employed KOt-Bu, because of the greater solubility of this base. We reasoned that this would also allow us to lower the reaction temperature, and thus use toluene as the solvent. We initiated a search for a suitable catalyst for bis-arylation of 4-benzylpyridine (1a) with bromobenzene (2a) by examining phosphine ligands on microscale using 10 μmol of 1a (see Supplementary Table 1). Thus, 48 electronically diverse mono- (20 mol%) and bidentate phosphine ligands (10 mol%) were tested using Pd(OAc)2 (10 mol%) and 4 equiv KOt-Bu. Reactions were conducted at 110 °C for 12 h, cooled, and quenched with water. The most promising microscale results, based on product to internal standard (4,4′-di-tert-butylbiphenyl) ratios, were with PCy3 (L1) and cataCXium A (L2, Fig. 2).
Figure 2. Ligands screening.
Preliminary reaction screen of 48 ligands.
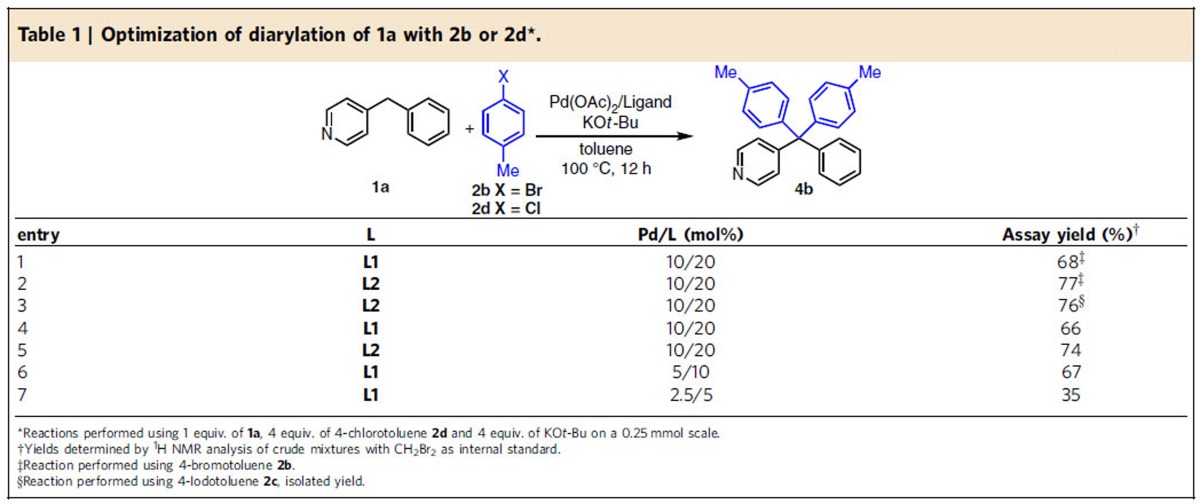
Repeating the bis-arylation reaction in Fig. 2 using 4-benzylpyridine 1a and 4-bromotoluene 2b with PCy3 (L1) and cataCXium A (L2) on laboratory scale (0.25 mmol) led to the desired product in 68 (PCy3) and 77% AY (cataCXium A) after 12 h (AY=assay yield, determined by 1HNMR analysis, see the Methods section for details) (Table 1, entries 1–2). With cataCXium A as the ligand, 4-iodotoluene 2c reacted with 4-benzylpyridine 1a to afford tetraarylmethane product in 76% isolated yield (entry 3). Considering that aryl chlorides are more abundant and less expensive than aryl bromides, we examined aryl chlorides. In the event, the reaction of 4-benzylpyridine 1a with 4-chlorotoluene 2d using the same conditions as above was conducted. Interestingly, the diarylated products were obtained in nearly identical assay yields with the aryl chlorides (66% PCy3 and 74% cataCXium A, entries 4–5) and aryl bromides. Although cataCXium A exhibited better AY than PCy3, we chose to first optimize the reaction with the more economical and readily available PCy3. As outlined below, more challenging substrates gave better results with cataCXium A. To begin with, the catalyst loading was examined. Changing the loading from 10 to 5 mol% resulted in no change in AY (entry 6). Further reducing the amount of catalyst to 2.5 mol%, however, resulted in a drop to 35% (entry 7).
Table 1. Optimization of diarylation of 1a with 2b or 2d*.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
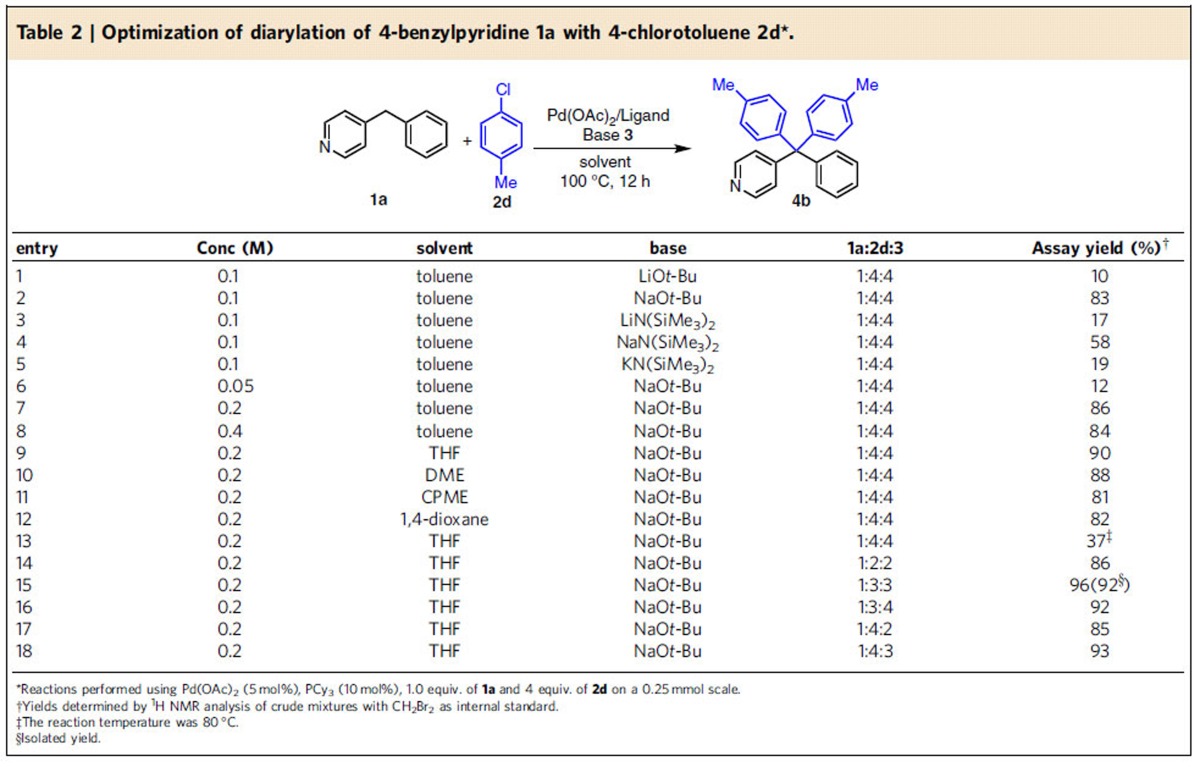
We next focused on the bases (LiOt-Bu, NaOt-Bu, LiN(SiMe3)2, NaN(SiMe3)2 and KN(SiMe3)2) and reaction concentration (Table 2, entries 1–8). LiOt-Bu and LiN(SiMe3)2 gave low yields (10–17%), probably because their stable aggregates result in less reactive bases (entries 1 and 3)31. NaN(SiMe3)2, and KN(SiMe3)2, are stronger bases, but resulted in some decomposition and lower yields (entries 4 and 5). The best combination from this screen was 5 mol% Pd(OAc)2, 10 mol% PCy3 and NaOt-Bu in toluene (0.2 M) for 12 h at 100 °C (entry 2). Solvents play an important role in deprotonative cross-coupling reactions. Therefore, four additional solvents (THF, DME, CPME and 1,4-dioxane) were examined at 100 °C. Among these, THF led to 90% AY of the diarylation products (entries 9–12). Reducing the reaction temperature to 80 °C resulted in a decrease in the AY to 37% (entry 13). The 4-benzylpyridine: 4-chlorotoluene: base ratio was next explored (entries 14–18). The use of a 1:3:3 ratio rendered 96% AY and 92% isolated yield (entry 15).
Table 2. Optimization of diarylation of 4-benzylpyridine 1a with 4-chlorotoluene 2d*.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
Diarylation of aryl(4-pyridyl)methanes
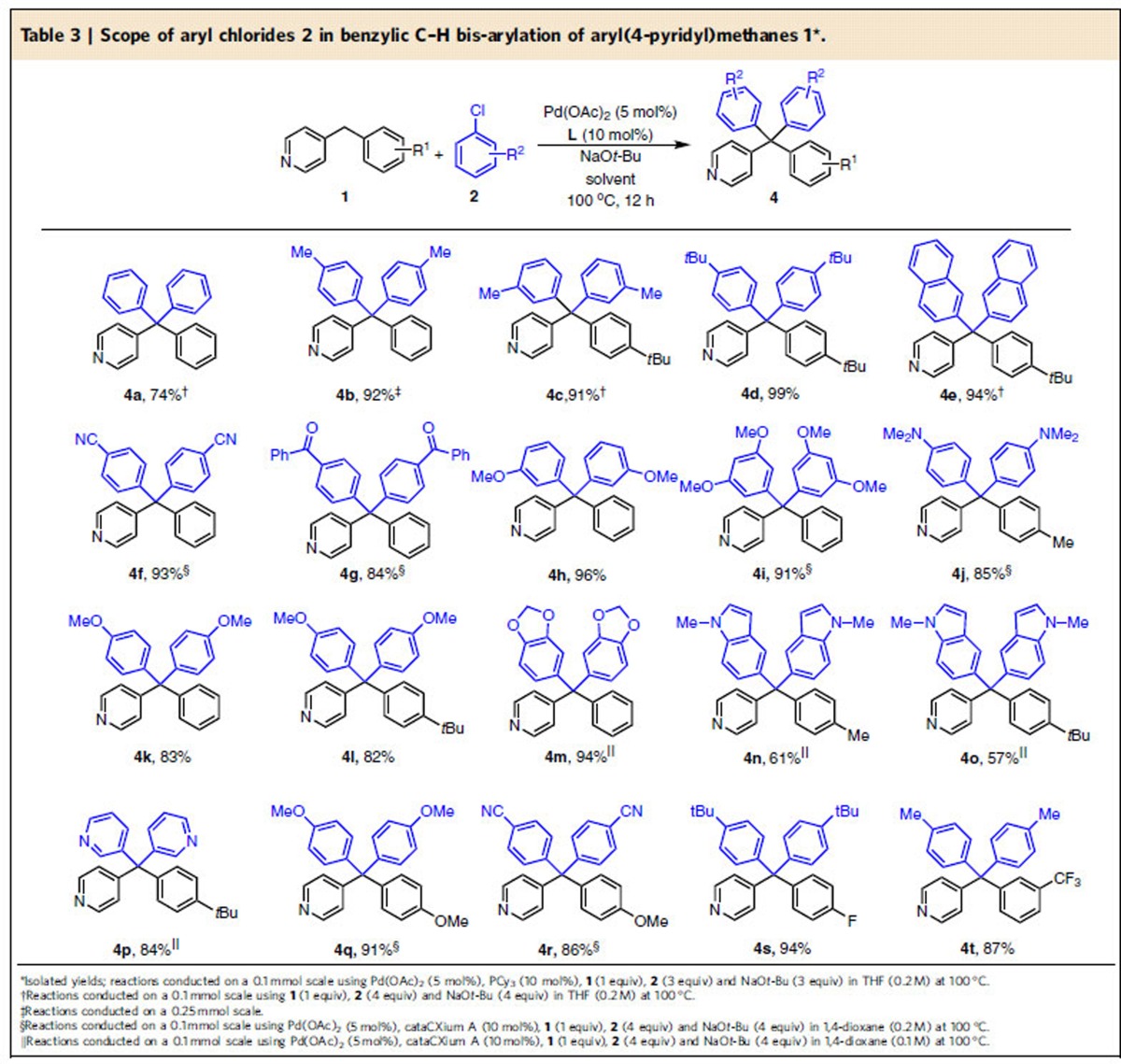
Under the optimized reaction conditions (Table 2, entry 15), the deprotonative cross-coupling process (DCCP) with various aryl(4-pyridyl)methanes and aryl chlorides generally gave products in good to excellent yields (Table 3). Coupling of 4-benzylpyridine and alkyl substituted derivatives with chlorobenzene or analogues possessing alkyl substituents in the meta or para positions resulted in products 4a–4e in 74–99% yield. Coupling with sensitive substrates, such as 4-chlorobenzonitrile and 4-chlorobenzophenone, required cataCXium A, furnishing 93 and 84% yield of 4f and 4g, respectively. In the absence of the palladium catalyst, 4-chlorobenzophenone gave 33% AY of the triarylmethane and no tetraarylmethane product. Using cataCXium A, reaction with 3-chloroanisole and 1-chloro-3,5-dimethoxybenzene provided the bis-arylated products 4h and 4i in 96 and 91% yield, respectively. Good yields were obtained using electron rich 4-chloro anisole (82–83%, 4k–l). Using 4-chloro-N, N-dimethylaniline under the standard conditions provided 4j in 85% yield (cataCXium A as the ligand). Heterocyclic compounds are present in many pharmaceuticals. The heterocyclic catechol derivative 4m formed in 94% yield (cataCXium A). With cataCXium A, 3-pyridylchloride and 5-chloro-1-methyl-1H-indole underwent reaction to give products 4n–4p in 57–84% yield. 4-Benzylpyridines possessing benzyl groups with 4-OMe, 4-F and 3-CF3 furnished products in 86–94% yield (4q–4t) (4q and 4r employed cataCXium A). It is noteworthy that many of these yields are high, despite undergoing two coupling events.
Table 3. Scope of aryl chlorides 2 in benzylic C–H bis-arylation of aryl(4-pyridyl)methanes 1*.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
Monoarylation of diaryl(heteroaryl)methanes
The ability to synthesize tetraarylmethanes with four different aryl groups would provide greater synthetic flexibility. Based on the successful bis-arylation of 4-benzyl pyridines above, we explored the monoarylation of diaryl(4-pyridyl)methanes 5 (Table 4). Triarylmethanes are readily prepared by arylation of diphenylmethane derivatives using our prior approach (see Supplementary Methods)32.
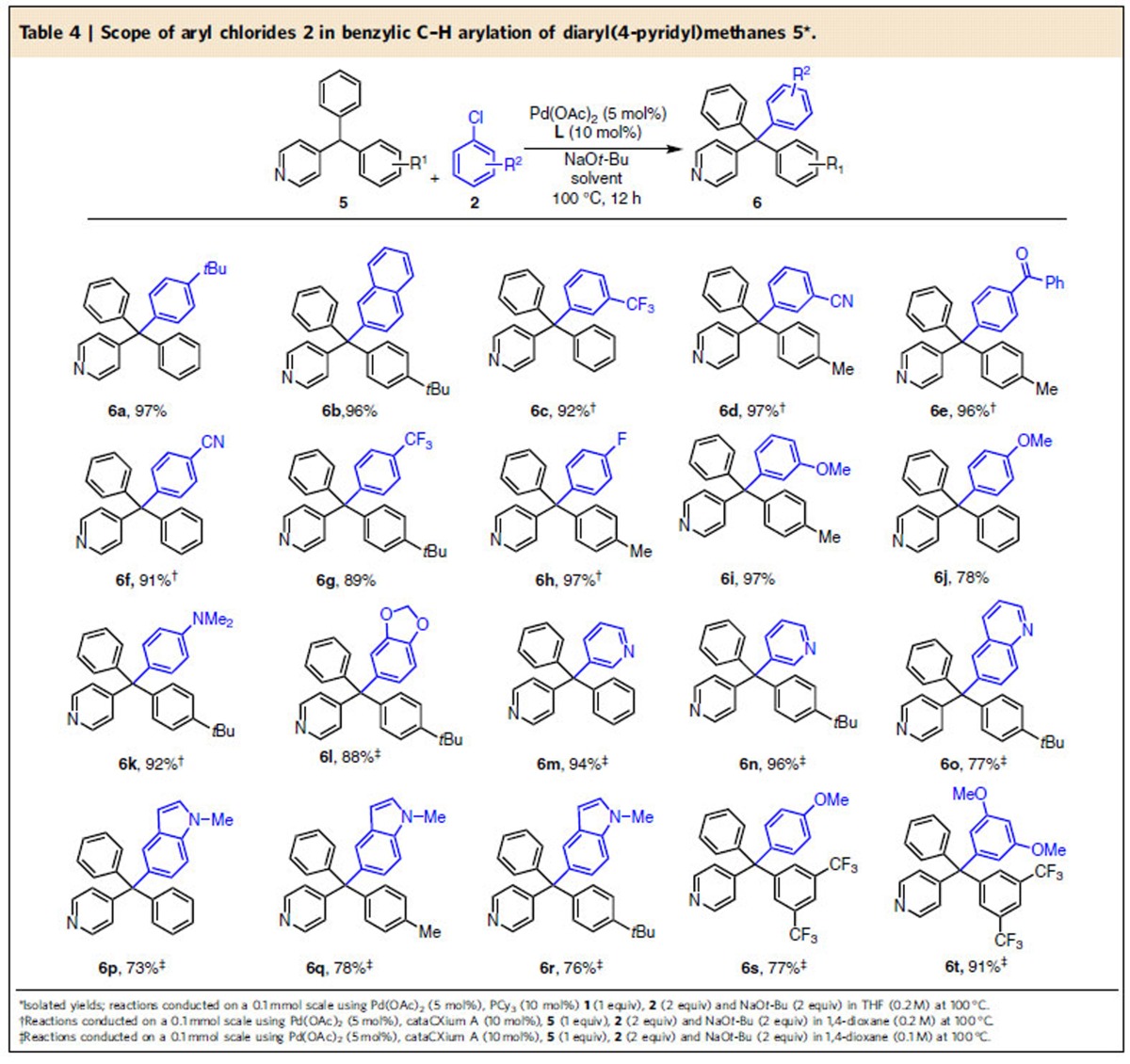
Table 4. Scope of aryl chlorides 2 in benzylic C–H arylation of diaryl(4-pyridyl)methanes 5*.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
Employing diaryl(4-pyridyl)methanes with two phenyl groups or one phenyl and one alkyl substituted aryl, aryl chlorides with alkyl or neutral groups furnished 6a and 6b in 97 and 96% yield, respectively. Aryl chlorides with 3-CN, 3-CF3, 4-COPh, 4-CN, 4-CF3, 4-F and 3-OMe reacted with 89–97% yield (6c–6i). Aryl chlorides bearing electron-donating groups 4-OMe and 4-NMe2, as well as catechol, underwent coupling in 78–92% yield (6j–6l). Heterocyclic aryl chlorides, including 3-pyridyl, 5-(N-methyl indole), and 6-quinolyl participated in the coupling with cataCXium A in 73–96% yield (6m–6r). Diaryl(4-pyridyl)methanes with a phenyl and 3,5-bis-CF3 aryl groups substituted aryl also participated in DCCP to produce the desired products 6s and 6t in 77 and 91% yield, respectively. In addition to the high yields generally observed in the synthesis of tetraarylmethane derivatives, it is noteworthy that many of the products contain chiral quaternary centres.
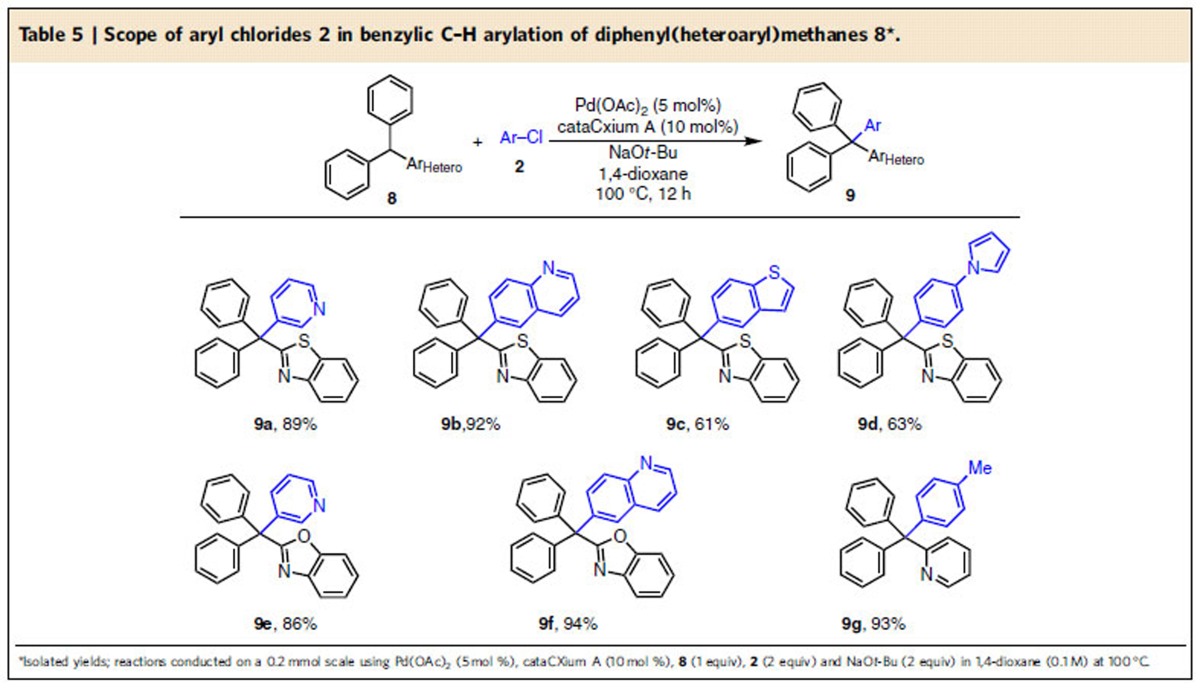
Due to the significance of heterocycles in drug discovery and in material science, we chose diphenyl(2-benzothiazolyl)methane (8a), diphenyl(2-benzoxazolyl)methane (8b) prepared from 2-methylthiazole (7a), 2-methylbenzoxazole (7b) using the literature approach24 (see General Methods A and Supplementary Methods) and diphenyl(2-pyridyl)methane (8c) to expand the scope of our tetraarylmethane synthesis (Table 5). Heteroaryl chlorides such as 3-chloropyridine and 6-chloroquinoline underwent coupling with diphenyl(heteroaryl)methanes (8), to furnish tetraarylmethane derivatives (9a, 9b, 9e and 9f) in high isolated yields (86–94%). In addition, 5-chlorobenzothiophene and 1-(4-chlorophenyl)-1H-pyrrole proved to be suitable aryl chlorides, furnishing products 9c and 9d in 61 and 63% yield, respectively. Diphenyl(2-pyridyl)methane coupled with 4-chlorotoluene to give 9g in 93% yield. With this catalyst system, less acidic diphenyl(3-pyridyl)methane did not afford the desired product under the optimized reaction conditions.
Table 5. Scope of aryl chlorides 2 in benzylic C–H arylation of diphenyl(heteroaryl)methanes 8*.
*Reaction conditions:1/LiHMDS/2/[Pd(η3-C3H5)Cl]2/S-IPr·HCl=200/200/100/2.5/5; 0.1 M of ketone 1; T=30oC; B/L and dr was determined by 1H NMR, dr is the ratio of (±)-(syn,anti)-3/other diastereoisomers; Isolated yield. †T=50 oC. ‡Solvent=THF. §OBoc of 2 was replaced with OP(OEt)2. ||The yield was determined by 1H NMR.
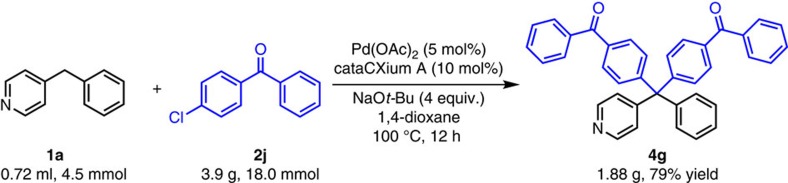
To demonstrate the potential utility of our method, we performed a gram scale reaction of 4-benzylpyridine 1a with 4-chlorobenzophenone 2j. The desired product 4g was obtained in 79% yield (1.88 g, Fig. 3), demonstrating the reaction is scalable (see Supplementary Methods).
Figure 3. Reaction on gram scale.
Diarylation of 4-benzylpyridine with 4-chlorobenzophenone on gram scale.
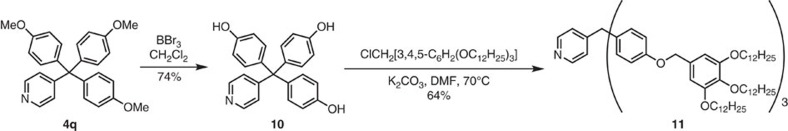
Kato and co-workers reported a liquid-crystalline bowl-shaped molecule that form columnar and micellar cubic structures, using triary(4-pyridyl)methane moieties as building blocks18. In order to apply our method, we synthesized the same compound (Fig. 4) from the reaction product 4q (Table 3). It is noteworthy that Kato synthesized triary(4-pyridyl)methane was based on Friedel-Crafts arylations from diaryl(4-pyridyl)methane with 46% yield, which is less than our method (91% yield, see Supplementary Methods).
Figure 4. Transformation of reaction product.
Synthesis of liquid crystal former 11.
Discussion
Our research team has been interested in the catalytic functionalization of weakly acidic sp3-hybridized C–H bonds through a DCCP. These reactions involve a weakly acidic C–H of the substrate (pronucleophile) that is reversibly deprotonated under the reaction conditions. Subsequently, the nucleophile undergoes catalyst promoted arylation33,34,35,36 or vinylation37. This method has been particularly successful with the generation of triarylmethanes38,39,40. Tetraarylmethane derivatives are challenging to efficiently prepare by both classical and state-of-the-art methods. The breadth of their applications has outpaced chemists' ability to prepare them in a concise fashion. Cross-coupling methods represent an appealing approach to tetraarylmethane derivatives, but to date successful reports of such processes are still lacking. Outlined herein is a palladium-catalysed DCCP for direct arylation of aryl(heteroaryl)methanes and diaryl(heteroaryl)methanes with aryl chlorides to provide triaryl(heteroaryl)methanes. Unlike traditional cross-coupling procedures, which employ prefunctionalized coupling partners, our approach relies on reversible deprotonation of the diarylmethane derivatives under the conditions used for the catalytic C–C bond forming reaction. Under our reaction conditions, a variety of triaryl(heteroaryl)methanes were prepared in good to excellent yields. This communication represents the first steps towards our goal of developing metal catalysed approaches for the construction of a wide range of tetraarylmethanes.
Methods
General procedure A
An oven-dried 8.0 ml reaction vial equipped with a stir bar was charged with 2-methylthiazole (7a, 0.50 mmol, 1.0 equiv) or 2-methylbenzoxazole (7b, 0.50 mmol, 1.0 equiv) and chlorobenzene (2e, 3.0 equiv) in a glove box under a nitrogen atmosphere at room temperature. A stock solution containing Pd(OAc)2 (5.6 mg, 0.025 mmol, 5 mol%) and PCy3 (14.0 mg, 0.05 mmol, 10 mol%) in dry o-xylene (2.5 ml). Then, NaOt-Bu (3.0 equiv) was added to the reaction mixture. The vial was capped, removed from the glove box, and heated to 130 °C for 12 h with stirring. The reaction mixture was quenched with 0.5 ml of H2O, diluted with 10 ml of ethyl acetate, and filtered over a pad of MgSO4 and silica. The pad was rinsed with ethyl acetate (10 × 2 ml) and the combined solutions were concentrated in vacuo. The crude material was loaded onto a deactivated silica gel column and purified by flash chromatography to afford the desired products 8a and 8b in 81 and 49% yield, respectively.
General procedure B
An oven-dried 8 ml reaction vial equipped with a stir bar was charged with aryl(4-pyridyl)methanes (1, 0.10 mmol, 1.0 equiv) or diaryl(heteroaryl)methanes (5, 0.10 mmol, or 8, 0.20 mmol, 1.0 equiv) and aryl chlorides (2, 2.0–4.0 equiv) in a glove box under a nitrogen atmosphere at room temperature. A stock solution containing Pd(OAc)2 (1.1 mg, 0.005 mmol, 5 mol%) and PCy3 (2.8 mg, 0.01 mmol, 10 mol%) in dry THF or cataCXium A (3.6 mg, 0.01 mmol, 10 mol%) in dry 1,4-dioxane was taken up by syringe and added to the reaction vial under nitrogen. Then, NaOt-Bu (2.0–4.0 equiv) was added to the reaction mixture. The vial was capped, removed from the glove box, and heated to 100 °C for 12 h with stirring. The reaction mixture was quenched with three drops of H2O, diluted with 3 ml of ethyl acetate, and filtered over a pad of MgSO4 and silica. The pad was rinsed with ethyl acetate (3 × 2 ml) and the combined solutions were concentrated in vacuo. The crude material was loaded onto a deactivated silica gel column and purified by flash chromatography to afford the desired products.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures and spectroscopic and physical data of compounds, see Supplementary Methods. For 1H and 13C{1H} NMR spectra of compounds, see Supplementary Figs 1–102.
Additional information
How to cite this article: Zhang, S. et al. Palladium-catalysed synthesis of triaryl(heteroaryl)methanes. Nat. Commun. 8, 14641 doi: 10.1038/ncomms14641 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures, supplementary table, supplementary methods and supplementary references.
Acknowledgments
We gratefully acknowledge the financial support from Nanjing Tech University and SICAM Fellowship from Jiangsu National Synergetic Innovation Center for Advanced Materials. We thank the National Science Foundation (CHE-1464744) and National Institutes of Health (NIGMS 104349) for financial support.
Footnotes
The authors declare no competing financial interests.
Author contributions S.Z. performed the experiments, B.-S.K. and J.M. performed the initial reaction development. C.W. performed screening experiments. P.J.W. devised the project and provided overall supervision. All authors contributed to writing the manuscript.
References
- Amabilino D. B., Ashton P. R., Belohradsky M., Raymo F. M. & Stoddart J. F. The self-assembly of branched [n] rotaxanes—the first step towards dendritic rotaxanes. J. Chem. Soc. Chem. Commun. 751–753 (1995). [Google Scholar]
- Ashton P. R. et al. A photochemically driven molecular-level abacus. Chem. Eur. J. 6, 3558–3574 (2000). [DOI] [PubMed] [Google Scholar]
- Dey S. K. et al. A redox-active reverse donor-acceptor bistable [2] rotaxane. Chem. Sci. 2, 1046–1053 (2011). [Google Scholar]
- Liao K.-Y. et al. Pt (II) metal complexes tailored with a newly designed spiro-arranged tetradentate ligand; harnessing of charge-transfer phosphorescence and fabrication of sky blue and white OLEDs. Inorg. Chem. 54, 4029–4038 (2015). [DOI] [PubMed] [Google Scholar]
- Dong J.-Q., Liu Y. & Cui Y. Chiral porous organic frameworks for asymmetric heterogeneous catalysis and gas chromatographic separation. Chem. Commun. 50, 14949–14952 (2014). [DOI] [PubMed] [Google Scholar]
- Lu J.-Z. & Zhang J. Facile synthesis of azo-linked porous organic frameworks via reductive homocoupling for selective CO2 capture. J. Mater. Chem. A 2, 13831–13834 (2014). [Google Scholar]
- Peschko K. et al. Dendrimer-type peptoid-decorated hexaphenylxylenes and tetraphenylmethanes: synthesis and structure in solution and in the gas phase. Chem. Eur. J. 20, 16273–16278 (2014). [DOI] [PubMed] [Google Scholar]
- Bonardi F. et al. Probing the SecYEG translocation pore size with preproteins conjugated with sizable rigid spherical molecules. Proc. Natl Acad. Sci. USA 108, 7775–7780 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X.-H. et al. Self-assembly morphology-tunable architectures from tetraarylmethane derivatives for targeted drug delivery. Langmuir 29, 3223–3233 (2013). [DOI] [PubMed] [Google Scholar]
- Brown D. G. & Boström J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone. J. Med. Chem. 59, 4443–4458 (2016). [DOI] [PubMed] [Google Scholar]
- Adams R., Hine J. & Campbell J. Triarylpyridylmethanes. J. Am. Chem. Soc. 71, 387–390 (1949). [Google Scholar]
- Grimm M., Kirste B. & Kurreck H. 13C-ENDOR investigation of an organic tetraradical in the quintet state; synthesis of a fourfold 13C-labeled tetrakisgalvinol. Angew. Chem. Int. Ed. Engl. 25, 1097–1098 (1986). [Google Scholar]
- Su D. & Menger F. M. Tetrastyrylmethane. Tetrahedron Lett. 38, 1485–1488 (1997). [Google Scholar]
- Zimmermann T. J. & Müller T. J. J. Convenient syntheses of tetraarylmethane starting materials. Synthesis 2002, 1157–1162 (2002). [Google Scholar]
- Ganesan P. et al. Tetrahedral n-type materials: efficient quenching of the excitation of p-type polymers in amorphous films. J. Am. Chem. Soc. 127, 14530–14531 (2005). [DOI] [PubMed] [Google Scholar]
- Xie L.-H. et al. Facile synthesis of complicated 9,9-diarylfluorenes based on BF3·Et2O-mediated Friedel-Crafts reaction. Org. Lett. 8, 3701–3704 (2006). [DOI] [PubMed] [Google Scholar]
- Plietzsch O. et al. Four-fold click reactions: generation of tetrahedral methane- and adamantane-based building blocks for higher-order molecular assemblies. Org. Biomol. Chem. 7, 4734–4743 (2009). [DOI] [PubMed] [Google Scholar]
- Hatano T. & Kato T. Nanostructured columnar and cubic liquid-crystalline assemblies consisting of unconventional rigid mesogens based on triphenylmethanes. Tetrahedron 64, 8368–8380 (2008). [Google Scholar]
- Schoepfle C. S. & Trepp S. G. The reaction between triarylmethyl halides and phenylmagnesium bromide. II. J. Am. Chem. Soc. 58, 791–794 (1936). [Google Scholar]
- Reetz M. T., Wenderoth B., Peter R., Steinbach R. & Westermann J. Efficient coupling of tertiary alkyl halides with dialkylzinc and titanium compounds. J. Chem. Soc., Chem. Commun. 1202–1204 (1980). [Google Scholar]
- Matsumoto K., Kannami M. & Oda M. Synthesis of tetrakis(2-pyridyl)methane: the first tetrapyridylmethane. Tetrahedron Lett. 44, 2861–2864 (2003). [Google Scholar]
- Nambo M. & Crudden C. M. Recent advances in the synthesis of triarylmethanes by transition metal catalysis. ACS Catal. 5, 4734–4742 (2015). [Google Scholar]
- Burton P. M. & Morris J. A. Palladium-catalyzed benzylic arylation of 2-methyl azaarenes. Org. Lett. 12, 5359–5361 (2010). [DOI] [PubMed] [Google Scholar]
- Song G.-D., Su Y., Gong X., Han K.-L. & Li X.-W. Pd(0)-catalyzed diarylation of sp3 C-H bond in (2-azaaryl)methanes. Org. Lett. 13, 1968–1971 (2011). [DOI] [PubMed] [Google Scholar]
- Xia Y.-X. et al. Palladium-catalyzed diarylmethyl C(sp3)-C(sp2) bond formation: a new coupling approach toward triarylmethanes. Org. Lett. 15, 1784–1787 (2013). [DOI] [PubMed] [Google Scholar]
- Niwa T., Yorimitsu H. & Oshima K. Palladium-catalyzed direct arylation of aryl(azaaryl)methanes with aryl halides providing triarylmethanes. Org. Lett. 9, 2373–2375 (2007). [DOI] [PubMed] [Google Scholar]
- Gartia Y., Biswas A., Stadler M., Nasini U. B. & Ghosh A. Cross coupling reactions of multiple C-Cl bonds of polychlorinated solvents with Grignard reagent using a pincer nickle complex. J. Mol. Catal. A–Chem. 363–364, 322–327 (2012). [Google Scholar]
- Cao X. et al. Palladium-catalyzed direct arylation of C−H bond to construct quaternary carbon centers: the synthesis of diarylfluorene. Org. Lett. 15, 3102–3105 (2013). [DOI] [PubMed] [Google Scholar]
- Chen J.-J. et al. Palladium-catalyzed arylation of methylene-bridged polyarenes: synthesis and structures of 9-arylfluorene derivatives. Adv. Synth. Catal. 354, 1551–1558 (2012). [Google Scholar]
- Nambo M., Yar M., Smith J. D. & Crudden C. M. The concise synthesis of unsymmetric triarylacetonitriles via Pd-catalyzed sequential arylation: a new synthetic approach to tri- and tetraarylmethanes. Org. Lett. 17, 50–53 (2015). [DOI] [PubMed] [Google Scholar]
- Bellomo A., Zhang J.-D., Trongsiriwat N. & Walsh P. J. Additive effects on palladium-catalyzed deprotonative-cross-coupling processes (DCCP) of sp3 C−H bonds in diarylmethanes. Chem. Sci 4, 849–857 (2013). [Google Scholar]
- Zhang J.-D., Bellomo A., Creamer A. D., Dreher S. D. & Walsh P. J. Palladium-catalyzed C(sp3)-H arylation of diarylmethanes at room temperature: synthesis of triarylmethanes via deprotonative-cross-coupling processes. J. Am. Chem. Soc. 134, 13765–13772 (2012). [DOI] [PubMed] [Google Scholar]
- McGrew G. I. et al. Asymmetric cross-coupling of aryl triflates to the benzylic position of benzylamines. Angew. Chem. Int. Ed. 51, 11510–11513 (2012). [DOI] [PubMed] [Google Scholar]
- Hussain N., Frensch G., Zhang J.-D. & Walsh P. J. Chemo- and regioselective C(sp3)-H arylation of unactivated allylarenes by deprotonative cross-coupling. Angew. Chem. Angew. Chem. Int. Ed. 53, 3693–3697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Kim B.-S. & Walsh P. J. Chemoselective palladium-catalyzed deprotonative arylation/[1,2]-Wittig rearrangement of pyridylmethyl ethers. Chem. Sci. 7, 976–983 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.-Y. et al. Palladium-catalyzed C-H arylation of α,β-unsaturated imines: catalyst-controlled synthesis of enamine and allylic amine derivatives. Angew. Chem. Int. Ed. 55, 2825–2829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-D., Kim B.-S., Li M.-Y. & Walsh P. J. Palladium-catalyzed selective α-alkenylation of pyridylmethyl ethers with vinyl bromides. Org. Lett. 18, 2371–2374 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-D. et al. Nixantphos: a deprotonatable ligand for room temperature palladium-catalyzed cross-coupling of aryl chlorides. J. Am. Chem. Soc. 136, 6276–6287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X.-Y. et al. Nickel-catalyzed arylation of heteroaryl-containing diarylmethanes: exceptional reactivity of the Ni(NIXANTPHOS)-based catalyst. Chem. Sci. 7, 611–618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-D. et al. Positional selectivity in C-H functionalizations of 2-benzylfurans with bimetallic catalysts. J. Am. Chem. Soc. 138, 4260–4266 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, supplementary table, supplementary methods and supplementary references.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. For the experimental procedures and spectroscopic and physical data of compounds, see Supplementary Methods. For 1H and 13C{1H} NMR spectra of compounds, see Supplementary Figs 1–102.