Table 3. Synthesis of difluorocyclopropyl allyl alcohols 17a–h from aldehydes 12a–h a .
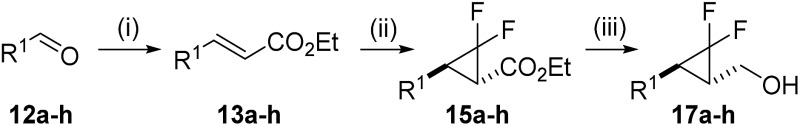
| ||||||
| Entry | R1 | x | 13 |
15
|
17 | |
| Yield b (%) | Conv. c (%) | Yield b (%) | Yield b (%) | |||
| 1 | 2-Furyl | a | 82 | 66 | 40 | 94 |
| 2 | Ph | b | — | 28 d | — | — |
| 3 | — | 29 d , e | n.d. f | — | ||
| 4 | 73 | 37 | n.d. f | — | ||
| 5 | 2-Thiophenyl | c | 98 | 77 | 71 | 50 |
| 6 | 5-Benzo[d][1,3]-dioxole | d | 94 | 50 | 43 | 75 |
| 7 | 2-Pyridyl | e | 87 | 0 | — | — |
| 8 | 2-N-Boc-pyrrolyl | f | 60 | 92 | 54 | 6 |
| 9 | 2-Thiazolyl | g g | 74 h | 0 | — | — |
| 10 | 3-Me-2-furyl | h i | 45 j | 80 | 45 | 84 |
a Conditions: (i) (carbethoxymethylene)triphenylphosphorane (1.1–1.3 eq.), DCM, r.t., 6–20 h (ii) MDFA (2.5 eq.), TMSCl (2.5 eq.), KI (2.8 eq.), diglyme (1.17 eq.), 120 °C, 4 h (iii) DIBAL (3 eq.), toluene or DCM, –78 °C to r.t., 8 h.
b Isolated yields.
c Determined by 1H NMR.
d Reaction time of 24 h.
e Starting from commercial ethyl cinnamate 13b.
f 13b and 15b were inseparable via column chromatography or distillation.
g Aldehyde 12g was synthesised from thiazole and used crude in the olefination reaction (see ESI for details).
h Calculated over two steps from thiazole, 4 : 1 mixture of E : Z-isomers.
i Aldehyde synthesised in situ from the oxidation of 2-hydroxymethyl-3-methyl furan (see ESI for details).
j Calculated over two steps from methyl 3-methyl-2-furoate.
