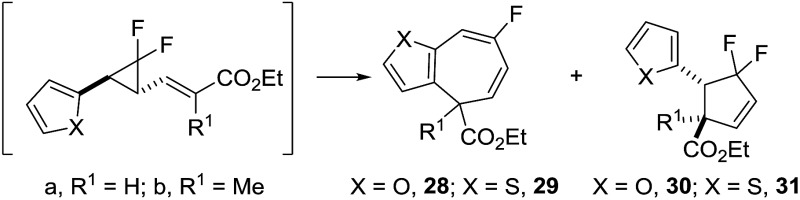
Table 5. Reaction outcomes from furyl and thiophenyl precursors 23–26 .
| |||||||
| Entry | VCP | X | R1 | Crude observations a (% conversion) | Product
b
(%) |
||
|
28/29
|
30/31 | ||||||
| 1 | 23 | O | H | 28a (>95), 30a (trace) | n.a. c | ||
| 2 | 24 | S | H | 29a major, 31a minor, evidence of 24 | 29a (12) | 31a (3) d | |
| 3 | 25 | O | Me | 28b (100) | 28b (48) | 30b (0) | |
| 4 | 26 | S | Me | 29b (100) | 29b (55) | 31b (0) | |
a Percentage conversion determined by 1H NMR after 1st purification.
b Isolated yield.
c Compound decomposed during purification attempts (see ESI for further information).
d 50% purity (determined by 1H NMR) containing 29a.
