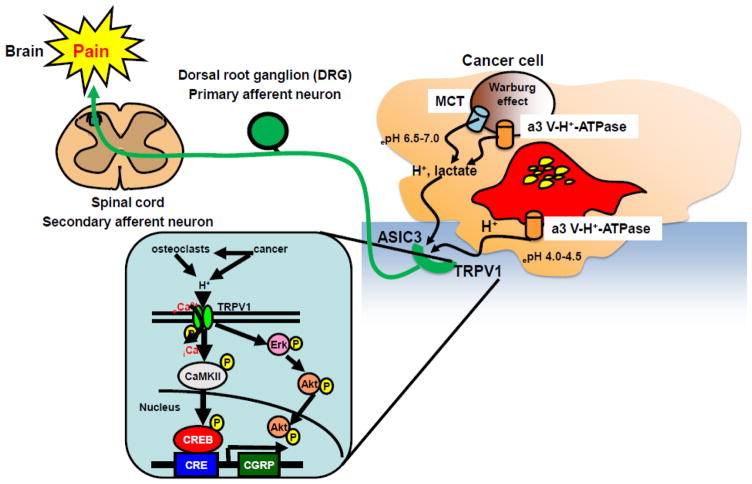
Figure 3. Acid-evoked cancer-associated bone pain (CABP).
Bone-resorbing osteoclasts secrete protons via plasma membrane a3 V-H+-ATPase to degrade bone minerals. Bone-colonizing cancer cells also release protons/lactate via MCT, a3 V-H+-ATPase and other proton pumps including Na+/H+ exchangers, anion exchangers, carbonic anhydrases, Na+/HCO3- co-transporters and HCO3-transporter to avoid intracellular acidification due to Warburg effect. The pH of the acidic environment created by cancer cells and bone-resorbing osteoclasts is assumed to be 6.5–7.0 [31] and 4.0–4.5 [23], respectively. The acidic microenvironment directly excites and sensitizes sensory neurons innervating bone via activation of the acid-sensing nociceptors, TRPV1 and ASIC3, transducing noxious signals to via DRG (primary afferent neuron) and spinal cord (secondary afferent neuron) and evoke bone pain in brain.
TRPV1 activation promotes eCa2+ influx into cytoplasm and induces propagation of intracellular signaling molecules including calmodulin kinase II (CaMKII) and the transcription factor cAMP responsive element-binding protein (CREB), leading to transcriptional activation of target molecules [52]. TRPV1 activation also propagates Erk and Akt presumably to prevent apoptosis of neuron cells. Blockade of TRPV1 activation under the acidic cancer environments may be a promising therapeutic approach to alleviate CABP.
ASIC3: Acid-sensing ion channel 3, CAMKII: Calmodulin kinase II, CGRP: calcitonin gene-related peptide, CREB: cAMP responsive element-binding protein, DRG: Dorsal root ganglion, MCT: Monocarboxylate transporter, TRPV1: Transient receptor potential channel-vanilloid subfamily member 1