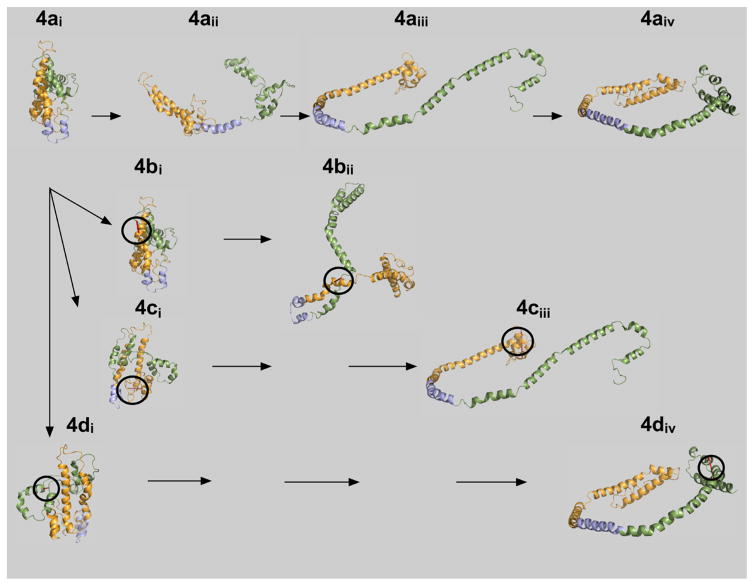
Figure 4.
Hypothetical lipid binding mechanism for ApoA-I. Lipid-free, wild-type apoA-I, 4ai, is compact and bundled such that the N- and C-terminal ends are folded and lie close to one another.24 Upon initial interaction of lipid with apoA-I, helical region 5 undergoes a transition to an extended conformation, 4aii. The hydrophobic core of apoA-I is now exposed, allowing central helices 4–6 to acquire lipid and form HDL particles, 4aiii and 4aiv. The resulting three-apoA-I particle carries up to 115 molecules of free cholesterol. Mutant apoA-Is are shown below wild-type apoA-I, 4ai. The circles indicate where the intramolecular disulfide bond is located in each of the mutants: F104C/H162C, 4bi, E34C/S55C, 4ci, and L200C/L233C, 4di. Note the unusual conformation forced upon the protein by the disulfide cross-link at F104C/H162C prevents helical regions 4–6 from opening to associate with hydrophobic lipids.