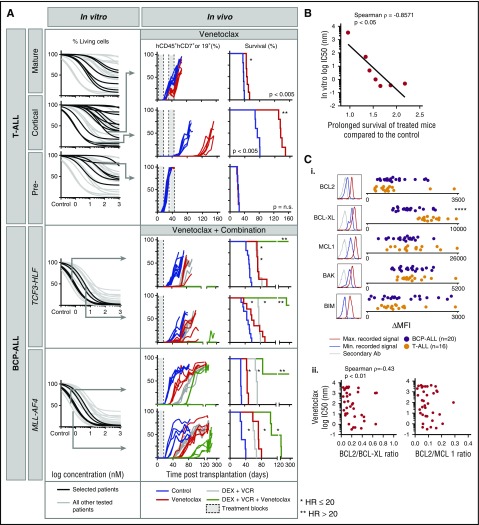
Figure 5.
In vitro sensitivity to the BCL-2 antagonist venetoclax correlates with the response in leukemia xenografts. (A) In vitro response to venetoclax for indicated ALL subtypes (black) compared with other ALL (gray). From top to bottom: mature T-ALL (n = 6), cortical T-ALL (n = 13), pre-T-ALL (n = 6), TCF3-HLF ALL (n = 4), and MLL-AF4 ALL (n = 3). Cell viability (7-aminoactinomycin D) was measured by flow cytometry after 72 hours of treatment and was normalized against controls treated with dimethyl sulfoxide. Arrows indicate samples whose response had been validated in vivo for venetoclax (top to bottom: T-VHR-03, T-HR-11, and T-HR-10) or venetoclax in combination with vincristine and dexamethasone (top to bottom: B-HR-24, B-HR-20, B-HR-26, and B-VHR-07). The left panel shows the number of leukemia cells compared with mouse lymphocytes over time. The right panel shows corresponding Kaplan-Meier survival curves (event defined as 25% of mCD45–hCD45+hCD19+ or hCD7+ leukemia cells detected by flow cytometry). (B) In vitro response to venetoclax correlates with fold increase of survival comparing treatment with venetoclax with treatment with vehicle (n = 7). (C) BCL2 protein family expression (i) analyzed by flow cytometer in T-ALL (n = 16) and BCP-ALL (n = 20). Correlation of BCL2:BCL-XL and BCL2:MCL1 ratio (ii) with in vitro venetoclax response. ****P < .001 (2-tailed Student t test). Ab, antibody; HR, hazard ratio; Max., maximum; MFI, mean fluorescent intensity; Min., minimum.