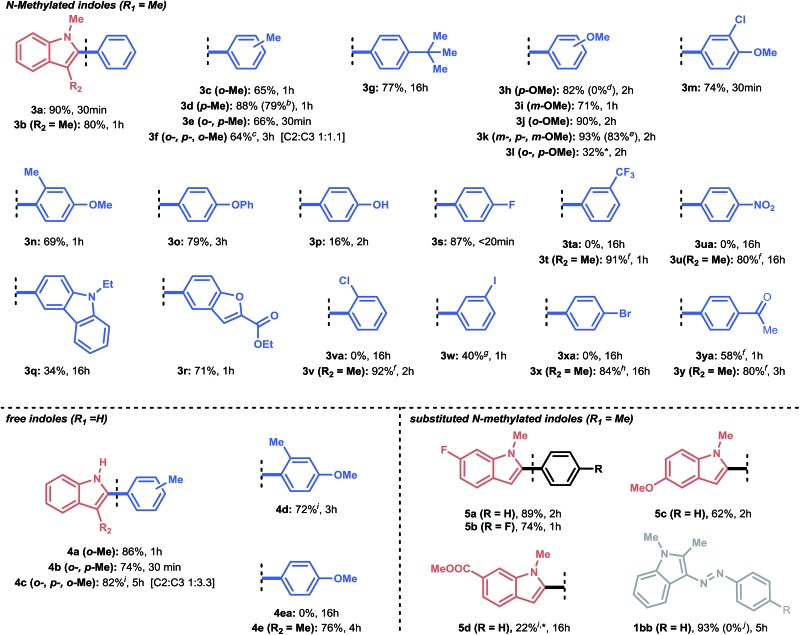
Table 2. Scope for the C-2 arylation of indoles a .
|
|
aReaction conditions: 0.5 to 1.0 mol% Pd(OAc)2, 1.0 mmol heteroarene and 1.0 equiv. aryldiazonium salt in 5 mL EtOAc : 2-MeTHF (1 : 1) at rt, open flask, 2 h premixing of Pd(OAc)2 with heteroarene.
bPd2(dba)3 as catalyst, 1 h reaction.
c1 mol% Pd(OAc)2, 1.2 equiv. aryldiazonium salt.
d4-Methoxybenzenediazonium mesylate was used.
eGram-scale experiment (10.0 mmol) yielded 2.47 g (83%), 4 h reaction time in 2-MeTHF as solvent.
f1 mol% Pd(OAc)2.
g2 mol% Pd(OAc)2.
h2 mol% Pd(OAc)2, 1.2 equiv. aryldiazonium salt.
i1.2 equiv. aryldiazonium salt at 40 °C; *no full conversion obtained.
j0.01 M and 100 mol% Pd2(dba)3 was used.
