Figure 3. Plasmid DNA is degraded more rapidly and remains primarily cytoplasmic in dendritic cells when compared to B lymphocytes.
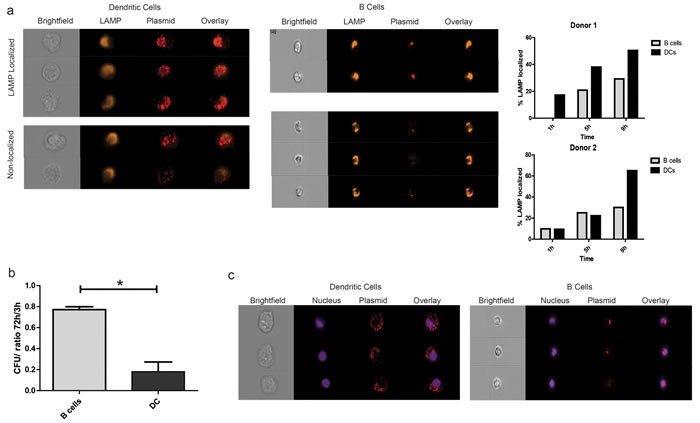
a. Enriched APC subsets were treated with fluorescently-labeled plasmid DNA as above and incubated for 1h, 5h, or 9h (1h in PBS, followed by culture in RPMI/10%AB media). Samples were then stained with a live dead marker and an antibody against the lysosome associated membrane protein (LAMP) and analyzed for intracellular localization by imaging cytometry at a 40X objective magnification. Shown are representative images of live CD11c+ and CD19+ cells that were plasmid positive after 9h that either co-localized with LAMP expression or not (left). Graphical representation of co-localization over time in two different donors as determined by the fraction of cells with a similarity-dilate score > 1 between the relevant fluorescence masks (right). b. Enriched APC subsets treated with pTVG4 were cultured for 3h or 72h and subjected to total nucleic acid extraction. Internal plasmid DNA was quantified by transformation of highly competent bacteria. Shown are number of colonies obtained from transformation with 100ng of total internal DNA, normalized to the number of cells of the particular type plated. c. Cell subsets were treated as in A, and stained with Hoechst 33342 prior to analysis. Cells with the highest plasmid nuclear localization scores were then retrieved using IDEAS 6.0, and visually verified for translocation into the nucleus. Shown are representative images of dendritic cells and B lymphocytes. Data are representative of 3 separate experiments, with at least 2 different donors.
