Figure 6. Cy-3-glu binds to LBD of ERα36 directly and inhibits its signaling pathway in TNBC cells.
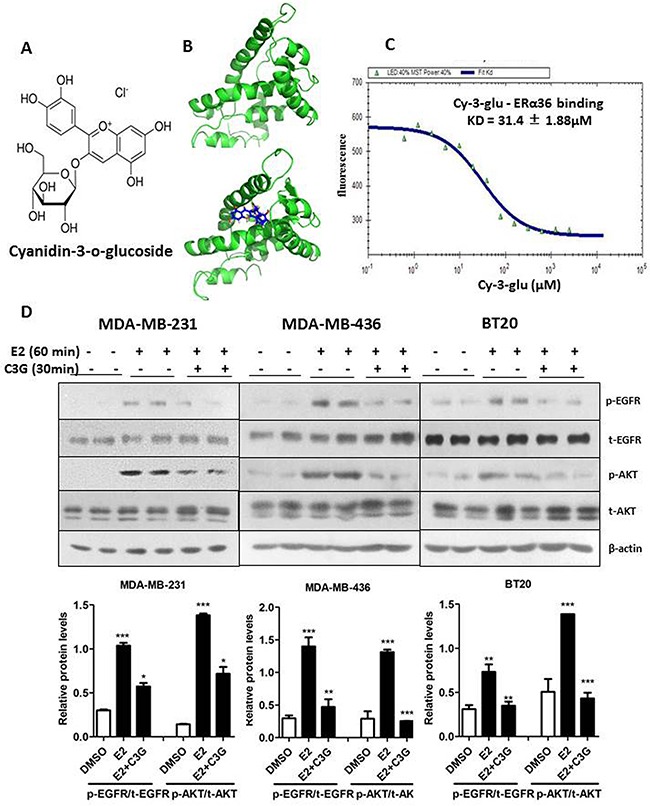
A. the chemical structure of Cy-3-glu. B. upper panel, a simulated docking model of the LBD-ERα36 (green color) complex. Lower panel, a simulated docking model of Cy-3-glu (dark blue color) in the pocket of LBD-ERα36 (green color). C. microscale thermophoresis analysis of Cy-3-glu binding to LBD-ERα36. Purified LBD-ERα36 protein was first labeled with Cy5/Alexa 647 fluorescence dye. Cy-3-glu was titrated between 0.61 and 2500 μM to the constant amount of labeled proteins (100 nM) and the binding affinity is 31.4 ± 1.88 μM. D. upper panel, phosphorylated-EGFR (p-EGFR), total EGFR (t-EGFR), p-AKT and t-AKT in TNBC cells were detected by Western blotting. Cy-3-glu (150 μM) for 30 min before E2 (100 nM) for 60 min. MDA-MB-231, MDA-MB-436 and BT20 cells were harvested and lysed. Lower panel, bar graphs show the relative protein levels of p-EGFR/t-EGFR and p-AKT/t-AKT. The immunoblots shown here are from a representative experiment repeated three times with similar results. Differences with p < 0.05 (*), p < 0.01 (**) or p < 0.001(***) are considered statistically significant.
