Figure 6. P2Y1 proteins physically interact with Pin1.
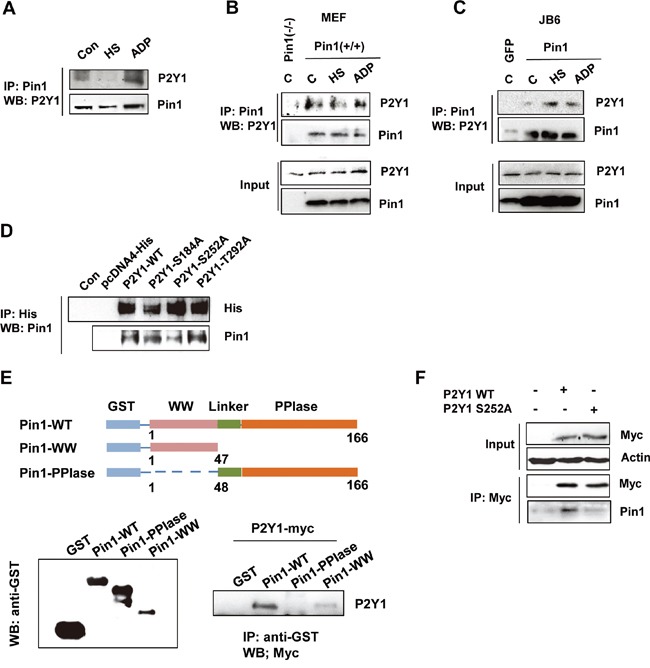
A. Endogenous Pin1 interacts with P2Y1 in hDPCs. hDPCs were subjected or not subjected to thermal stress or treated or not treated with ADP for 24 h. hDPCs were lysed and used for immunoprecipitation (IP) with anti-Pin1 antibody. Pin1 or P2Y1 was visualized by immunoblotting. B, C. Pin1 strongly interacts with P2Y1 in MEF Pin1+/+ and JB6-Pin1 cells. Pull-down experiments were performed as described above. A total of 100 μg of total protein from the lysate pull-down products (top panel) and inputs (bottom panel) were subjected to IB analysis with P2Y1 and Pin1 antibodies. D. The S252 residue of P2Y1 mediates the Pin1–P2Y1 interaction. Three residues (S184, S252, and T292) were mutated to alanine. H1299 cells were transfected with either P2Y1 wild-type or mutant expression plasmids. The cells were lysed and subjected to a pull-down assay. Immunoblotting analysis was performed using an antibody specific for His and Pin1. E. P2Y1 binds to the WW domain but not the PPIase domain of Pin1. Schematic representation of full-length Pin1 and various truncation mutants. Whole-cell lysates derived from HEK293 cells expressing myc-tagged P2Y1 were incubated with glutathione-Sepharose beads containing GST, GST-Pin1-WT, GST-Pin1-WW, or GST-Pin1-PPIase. After washing, the bound proteins were subjected to immunoblot analysis with anti-myc antibody. F. hDPCs were transfected with either P2Y1 wild-type or P2Y1 S252 plasmids. The cells were lysed and subjected to a pull-down assay. Immunoblotting analysis was performed using antibodies specific for myc and Pin1.
