Figure 2. HIC1 SUMOylation is not required for efficient DSBs repair.
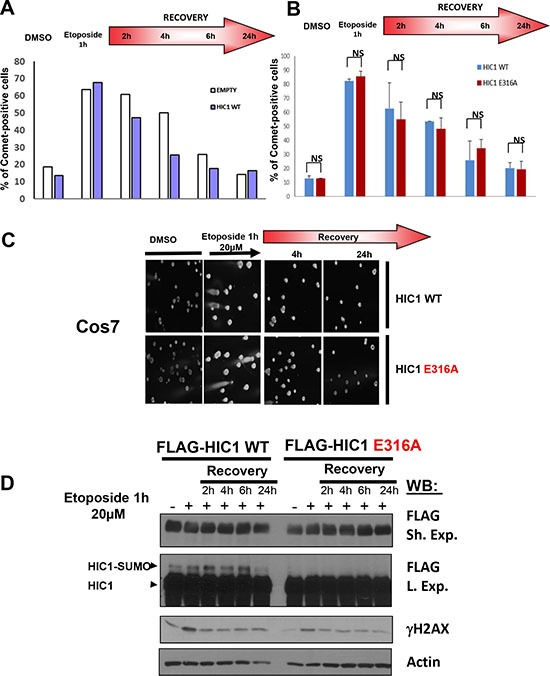
(A) Cos7 cells were transfected for 48 hours with wt FLAG-HIC1 or with the empty pcDNA3FLAG expression vector. Cells were either mock-treated with DMSO (–) or treated with 20 μM etoposide (+) for 1 hour. After removal of the drug, cells were allowed to recover in normal medium for various times (2, 4, 6 and 24 hours) and DSBs were monitored by neutral Comet assay. The percentage of Comet positive cells reflecting unrepaired DNA breaks is depicted after counting at least 100 cells in each condition. (B) Cos7 cells were transfected for 48 hours with wt FLAG-HIC1 or with the non-SUMOylatable E316A point mutant. Neutral Comet assays were performed and analyzed as described in panel A). The error bar indicates mean +/– standard deviation of three independent experiments (NS: not significant). (C) Representative Comet images of mock-treated (DMSO) and of cells treated with etoposide for 1 hour after transfection of wt HIC1 or of E316A HIC1 with or without recovery in normal medium for 4 and 24 hours, respectively. (D) Western blot analyses of cells transfected with wt HIC1 or with E316A HIC1 Samples of cells in each condition were taken before the Comet assays and immediately lysed in Laemmli loading buffer. These whole cell extracts were analyzed by Western blot with anti-FLAG antibodies to detect HIC1 and its SUMOylated forms. γH2AX and actin levels were used as controls for DSB induction and equal loading, respectively.
