Figure 5. At least two domains in the C-terminal end of MTA1 are implicated in the interaction with HIC1.
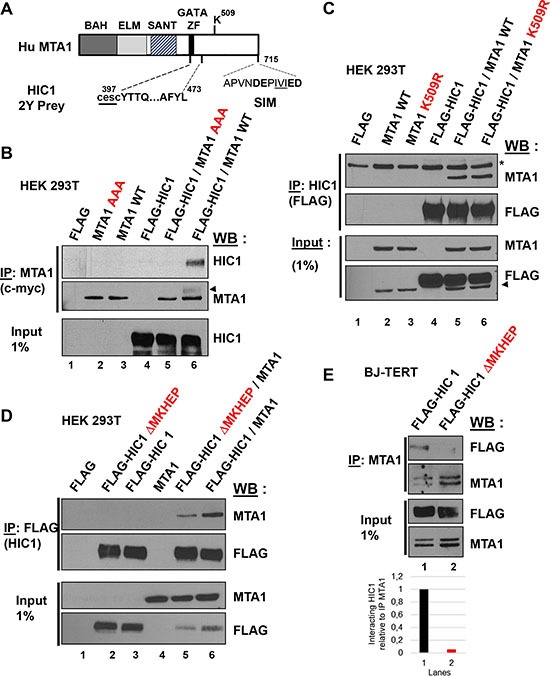
(A) Schematic drawing of the human MTA1 protein. The domains identified in MTA1 include the BAH (Bromo-associated homology), the ELM (Egl-27 and MTA1 homology), the SANT (SW13, ADA2, N-CoR and TF1118) and the GATA-like zinc finger. The region isolated in the two-hybrid screen with HIC1 is shown with the two first cysteines of the GATA zinc finger not present in the isolated prey, shown as lower-case letters underlined [11]. The Lysine 509, which is SUMOylated, and the C-terminal SIM motif are also shown with the hydrophobic core, IVI, underlined and the flanking acidic residues in bold [19]. (B) The SIM (SUMO-interacting motif) in the C-terminal end of MTA1 is required for its interaction with HIC1. After transfection with the indicated expression vectors, HEK293T cells lysates were immunoprecipitated with anti-c-myc antibodies. Immunoprecipitated samples [IP c-myc (MTA1)] and 1% of whole cell extracts (Input) were analyzed by immunoblotting with anti-HIC1 antibodies to detect co-immunoprecipitation. To control for IP efficiency, the membrane was stripped and probed with anti-MTA1 antibodies (the arrow head indicates a remnant of the HIC1 band). (C) SUMOylation of MTA1 on Lysine K509 is not required for the HIC1-MTA1 interaction. A similar experiment was performed in HEK293T with expression vectors for wt MTA1 or its non-SUMOylatable version (K509R) and HIC1. In the top panel, *refers to a non-specific band detected. In the bottom panel, the arrowhead indicates a remnant of the MTA1 band. (D) The HIC1-MTA1 interaction is also strongly reduced by deletion of the HIC1 SUMOylation motif, ΔMKHEP. A similar Co-IP experiment was realized in HEK293T but with expression vectors for the wt FLAG-HIC1 or the FLAG-HIC1 ΔMKHEP deletion mutant and wt MTA1. (E) Interaction of wt and ΔMKHEP HIC1 with endogenous MTA1 proteins in HEK293T cells. Total extracts of HEK293T transfected with the indicated plasmids were analysed by Co-IP with anti-MTA1 antibodies and immunoblotted with MTA1 and FLAG antibodies. Note that the endogenous MTA1 proteins in the immunoprecipitated materials or in the Inputs migrate as a doublet.
