Figure 3. Different models of activation of tyrosine phosphorylation during capacitation.
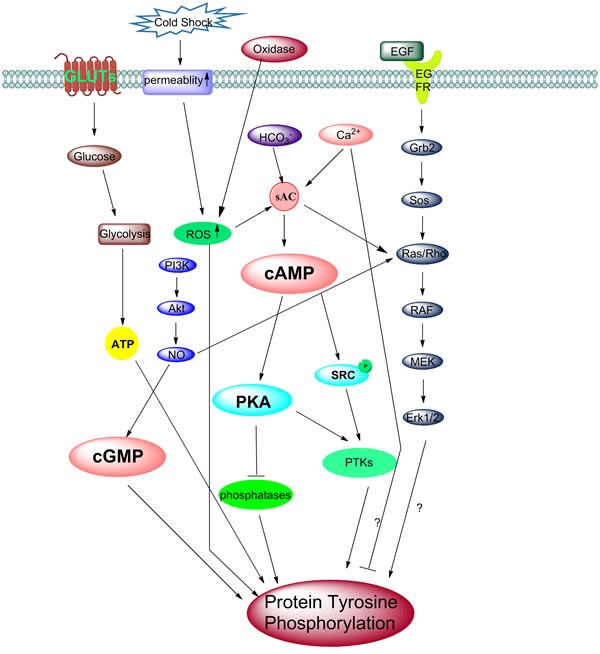
A schematic representation of the different molecular models of the activation of tyrosine phosphorylation during sperm capacitation. The removal of cholesterol from the plasma membrane increases membrane fluidity, results in an influx of HCO3- and Ca2+ ions through NBC and calcium channels. The increased intracellular concentration of HCO3-, Ca2+ and ROS, activates the sAC/PKA pathway. However, several papers also report Ca2+ negatively regulates protein tyrosine phosphorylation in human sperm. These phosphorylation of PKA substrates might be directly or indirectly involved in the phosphorylation of MEK-like proteins and subsequently tyrosine residues in fibrous sheath proteins. Also, Src family kinases can inactivate phosphatase, then change the PKA phosphorylation status. Interestingly, cold shock can stimulate and enhance the permeability of the membrane, which also stimulates the ROS pathway and causes an increase in protein tyrosine phosphorylation (PTP). The onset of tyrosine kinases activation is followed by tyrosine phosphorylation. The binding of EGF activates the ERK pathway which increases PTP. NO· can activate the ERK pathway intermediate Ras/Rho protein, while a high NO· can also up act to regulate the cGMP/PKG pathway. The PI3K/Akt axis always modulates the phosphorylation of the Thr-Glu-Tyr motif and tyrosine. It is possible that the downstream effectors such as PDK1 and Akt activate NOS, which then stimulate Ras and the ERK pathway and later cause the increase in PTP. ROS may involve in this pathway as well. Besides, another important factor is glucose, transported by GLUTs into the sperm cell, are useful for ATP generation by glycolysis. ATP is then used for sperm hyperactivation motility and PTP.
