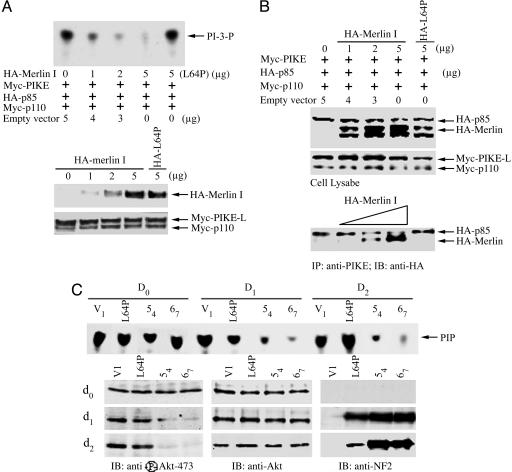
Fig. 3.
merlin blocks the stimulatory effect of PIKE-L on PI3-kinase. (A) TLC was used to assay PI3-kinase activity. HEK293 cells were transfected with the indicated expression constructs. PI3-kinase was immunoprecipitated by anti-p110 antibody and assayed for in vitro lipid kinase activity. (Top) Increasing amount of merlin progressively diminished PI3-kinase activity, but L64P failed to do so. (Middle) Gradually increasing levels of HA-merlin was expressed in transfected cells. (Bottom) Equal amount of Myc-p110 and Myc-PIKE-L was confirmed by anti-myc immunoblotting. (B) merlin competes with PI3-kinase for binding to PIKE-L. (Bottom) Myc-PIKE-L was immunoprecipitated by anti-PIKE antibody, and the coprecipitated HA-p85 and HA-merlin were visualized by Western blot with anti-HA antibody. (Middle) Equal amounts of Myc-p110 and PIKE-L were confirmed with anti-Myc antibody. (Top) Increased expression of merlin was confirmed. (C) Merlin mediates PI3-kinase in schwannoma cells. TLC was used to assay PI3-kinase activity. Rat RT4-D6P2T schwannoma cells stably transfected with empty vector, merlin mutant L64P, or WT merlin (54 or 67) were uninduced or induced with doxycycline for 0, 1, or 2 days. (First blot from top) PI3-kinase activity is abolished by induced WT merlin, but not merlin mutant L64P. Akt and its phosphorylation in the lysate of schwannoma cells were analyzed. (Left, lower three blots) WT, but not patient-derived L64P, merlin blocks Akt phosphorylation. (Center and Right, lower three blots) The expression of Akt and induced merlin was verified.