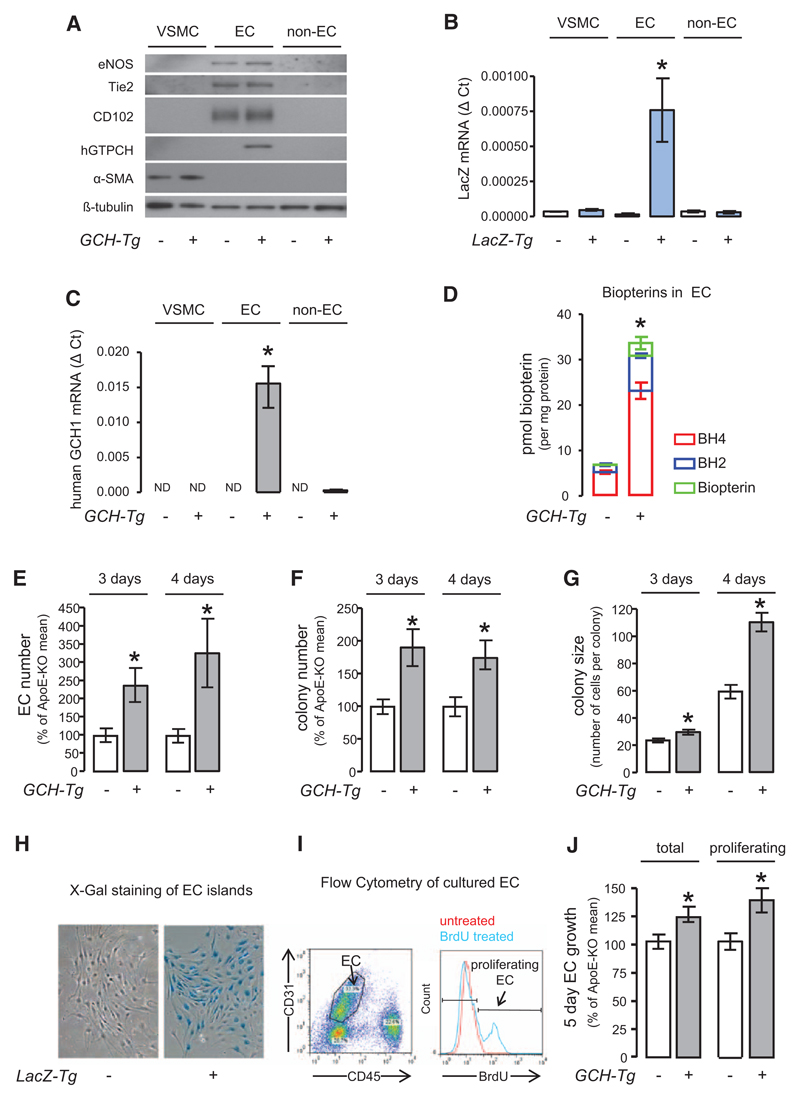
Figure 2. Endothelial-specific overexpression of human GTP cyclohydrolase (GCH) increases endothelial biopterin levels and promotes in vitro growth of primary endothelial cells (ECs).
A, Western blotting showed that the EC markers endothelial nitric oxide synthase (eNOS), Tie-2, and CD102 were exclusively expressed in ECs. B, Reverse transcription polymerase chain reaction showed that the expression of human GCH mRNA was specific to GCH transgenic EC (unpaired t test with Welch’s correction *P<0.05 GCH transgenic EC [n=3] vs GCH transgenic non-EC [n=3], no human GCH1 detectable in vascular smooth muscle cell [VSMC] or apolipoprotein E [apoE]-knockout [KO] cells [n=4 each]). C, Expression of LacZ mRNA was specific to LacZ transgenic ECs (unpaired t test with Welch’s correction *P<0.05 LacZ transgenic EC [n=3] vs LacZ transgenic non-EC [n=3] and VSMC [n=4]). D, Biopterin levels in GCH/apoE-KO EC were increased by ≈5 fold compared with apoE-KO. Unpaired t test *P<0.001 GCH/apoE-KO (n=4) vs apoE-KO (n=4). Primary ECs were isolated from apoE-KO/LacZ and GCH/apoE-KO/LacZ animals and grown in tissue culture. The total number of EC (E), the number of EC colonies (F), and average colony size (G) were significantly increased in GCH/apoE-KO cultures both after 3 and 4 days in culture assessed by X-galactosidase (H). Unpaired t test with Welch’s correction *P<0.05 GCH/apoE-KO vs apoE-KO (n=5 per group for 3-day culture, n=6 per group for 4-day culture). Flow cytometry analysis of EC growth in culture. To identify proliferating EC, Bromodeoxyuridine (BrdU) was added to the culture media. I, Both total number of EC and number of proliferating EC were significantly increased in GCH/apoE-KO (J). Unpaired t test *P<0.05 GCH/apoE-KO vs apoE-KO (n=9 per group).