Abstract
Regular physical exercise reduces the risk of cardiovascular disease and improves outcome in patients with cardiovascular diseases. The dynamic changes in blood pressure and heart rate with acute exercise are independently predictive of prognosis. Quantification of the haemodynamic response to exercise training in genetically modified mouse models may provide insight into the molecular mechanisms underlying the beneficial effects of exercise. We describe, for the first time, the use of radiotelemetry to provide continuous blood pressure monitoring in C57BL/6J mice during a programme of voluntary wheel exercise with continuous simultaneous recording and analysis of wheel rotations and beat-by-beat haemodynamic parameters. We define distinct haemodynamic profiles at rest, during normal cage activity and during episodes of voluntary wheel running. We show that whilst cage activity is associated with significant rises both in blood pressure and in heart rate, voluntary wheel running leads to a further substantial rise in heart rate with only a small increment in blood pressure. With 5 weeks of chronic exercise training, resting heart rate progressively falls, but heart rate during episodes of wheel running initially increases. In contrast, there are minimal changes in blood pressure in response to chronic exercise training. Finally, we have quantified the acute changes in heart rate at the onset of and recovery from individual episodes of wheel running, revealing that changes in heart rate are extremely rapid and that the peak rate of change of heart rate increases with chronic exercise training. The results of this study have important implications for the use of genetically modified mouse models to investigate the beneficial haemodynamic effects of chronic exercise on blood pressure and cardiovascular diseases.
Measures of autonomic dysfunction during exercise are an important quantitative predictor of cardiovascular risk and prognosis in man (Aktas et al. 2004; Balady et al. 2004; Erikssen et al. 2004). Regular physical exercise is associated with improved autonomic balance and is an important protective factor in long-term cardiovascular health (Tanasescu et al. 2002). Beneficial effects of exercise have been demonstrated in hypertension (Kelley & Kelley, 2000; Whelton et al. 2002), ischaemic heart disease (Jolliffe et al. 2001; Thompson et al. 2003; Taylor et al. 2004), myocardial infarction (Dorn et al. 1999) and heart failure (Belardinelli et al. 1999; Piepoli et al. 2004). Acute exercise in humans leads to a rapid increase in both heart rate and arterial blood pressure, which is closely correlated with the intensity of exercise (Sharman et al. 2005). In contrast, chronic regular exercise training lowers resting heart rate and blood pressure whilst accelerating heart rate recovery following exercise, itself an independent predictor of prognosis (Cole et al. 1999, 2000; Nishime et al. 2000; Shetler et al. 2001; Aktas et al. 2004; Jouven et al. 2005). Identification of the specific molecular and genetic mechanisms underlying the autonomic consequences of exercise offers potential for the identification of future therapeutic targets in humans (Gielen et al. 2010). Although genetically modified mouse models have considerable potential to provide insight into these mechanisms, accurate phenotyping of the murine autonomic response to exercise has been technically difficult.
Little is known about the normal murine blood pressure and heart rate response to acute and chronic exercise in physiological conditions. Previous attempts have been limited to the use of tethered or telemetered animals undergoing forced treadmill exercise, usually over short time periods (Desai et al. 1997; Rohrer et al. 1998; Masuki et al. 2003; Schuler et al. 2010). Interpretation of such experiments is difficult owing to the use of a forced exercise regime and/or the lack of adequate postoperative recovery time following chronic arterial cannulation, both of which are likely to induce a significant and confounding stress response (Van Vliet et al. 2000). In contrast, voluntary exercise has the advantage of being physiological and non-stressful and does not interfere with normal murine diurnal rhythms. When provided with a running wheel, C57BL/6 mice will run long distances spontaneously (Swallow et al. 1998; Allen et al. 2001; Lerman et al. 2002; Bernstein, 2003; Konhilas et al. 2004; De Bono et al. 2006). Assessment of the blood pressure and heart rate response to voluntary exercise requires continuous monitoring over long periods of time using techniques that do not impact adversely on the ability to exercise.
Recent advances in radiotelemetry (Mills et al. 2000; Butz & Davisson, 2001; Van Vliet et al. 2003) allow continuous real-time measurement of arterial blood pressure and heart rate in ambulant, non-restrained, unanaesthetized mice, in normal cage conditions. Radiotelemetry overcomes many of the limitations of more traditional approaches, such as tail-cuff (Krege et al. 1995; Van Vliet et al. 2003) or tethered systems (Davisson et al. 1997; Mattson, 1998); however, its use to investigate the blood pressure responses to exercise in mice has been limited by potential effects of the size of the device on exercise performance and by technical difficulties in precisely correlating beat-to-beat haemodynamic changes with exercise episodes.
A new murine radiotelemeter (PAC10 DSI®) has recently been developed, which is 50% smaller by weight and volume than previous devices (PAC20 DSI®). We have combined use of this smaller telemeter with a system allowing continuous, real-time monitoring of voluntary wheel running in mice (De Bono et al. 2006) enabling, for the first time, the production of a simultaneous recording of wheel running and beat-to-beat changes in blood pressure. We now report the acute haemodynamic response of C57BL/6J mice to different intensities of voluntary exercise and the effect of chronic exercise training on these parameters in vivo.
Methods
Animals
All animals were 10- to 12-week-old male C57BL/6J strain littermates from an inbred colony (mean weight 29–30 g). Mice were provided with standard chow and water ad libitum and housed singly at 24°C in individually ventilated cages (Techniplast Inc., Milan, Italy). All mice were exposed to a regular 12 h–12 h light–dark cycle. Studies were performed in accordance with both the UK Home Office Animals (Scientific Procedures) Act 1986 and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). The study was approved by the Local Ethical Approval Panel.
Implantation of radiotelemeters
The PAC10 radiotelemeters (DSI, Transoma Medical Inc. St Paul, MN, USA) were implanted in 10- to 12-week-old C57BL/6 mice under carefully titrated isofluorane anaesthesia adjusted to ensure abolition of established murine pain reflexes, including the pedal withdrawl reflex. Mice were kept on a warming blanket and eye protection provided with Viscotears. The surgical field was sterilized with chlorhexidine and the procedure performed under an extraction hood in full sterile conditions. Buprenorphine (0.02 mg) was administered to provide postoperative analgesia. Telemeter catheters were implanted in the left carotid artery, with the body of the telemeter placed in a subcutaneous pocket equidistant from the fore- and hindpaw (Mills et al. 2000; Butz & Davisson, 2001). The wound was then closed with 4.0 Vicryl. Postoperatively, mice were held in a recovery chamber at 37°C until mobile and subsequently moved to a recovery cabinet at 28°C for a further 4 h. Animals were kept under close observation for the duration of the experiment, both by the experimental team and by the veterinary officer. Additional buprenorphine and subcutaneous 0.9% normal saline were given where necessary, although recovery was usually rapid. All mice underwent 14 days postoperative recovery before commencement of voluntary running. Blood pressure traces were checked, and animals with damping of the haemodynamic profile were excluded from analysis. On completion of the experiment, animals were killed by terminal isofluorane anaesthesia.
Haemodynamic measurements
The PAC10 radiotelemeters allow continuous monitoring of blood pressure waveforms and cage activity. Data were acquired continuously at 500 Hz using standard acquisition software (DSI, Transoma Medical Inc.). Periods of recording were carried out over 24 h for the duration of exercise training. Recordings were taken daily for the first 5 days of wheel activity but subsequently, in order to preserve battery life, on at least alternate days.
Voluntary exercise
The system used for monitoring voluntary running in mice individually housed in individually ventilated cages has been described previously (De Bono et al. 2006). In brief, following surgical recovery the mice were introduced to a freely rotating angled running track (Lillico Inc., Surrey, UK). Wheel rotations were monitored using an optical switch mounted outside the cage capable of recording the passage of a stainless-steel mirror attached to the external rim of the running track. The exact timing of individual wheel rotations was logged using Spike2 acquisition software (Cambridge Electronic Design Ltd, Cambridge, UK). Mice were allowed to use the wheel ad libitum and were monitored continuously for the duration of the period of exercise training.
Data analysis
Analysis of both wheel rotations and haemodynamic data were performed using Spike2 software (Cambridge Electronic Design Ltd). Raw blood pressure waveform data were imported into Spike2 and merged with the wheel rotation data corresponding to each animal for a 24 h period. This allowed a precise time correlation between wheel rotations and haemodynamic parameters on a beat-to-beat basis. Data were analysed according to the activity state of the animal at the time of acquisition. The following three activity states were defined: wheel running, defined as periods of continuous wheel rotation with a maximal rotation-to-rotation interval of 5 s; cage activity, characterized by a lack of wheel rotations but movement within the cage defined by the presence of a signal on the DSI activity trace; and rest, defined as periods with no wheel rotations and no signal on the DSI activity trace preceded and succeeded by at least 30 activity-free seconds.
Haemodynamic profiles for systolic, mean and diastolic blood pressure, pulse pressure and heart rate were determined for each activity state for each day of recording for the duration of the period of exercise training. A rolling 3 day average was then performed for each parameter to allow interpolation of battery-life-preserving ‘off days’ prior to final analysis.
For onset and offset heart rate analysis, running bouts were defined as periods of wheel rotation of at least 30 s duration preceded or succeeded by at least 60 s with no further wheel rotations. A sigmoid curve fitted to the heart rate was then constructed at the point of each marked onset or offset. The heat rate data from the analysable curves for each 24 h period were then averaged around the mid-point of the sigmoid curve to obtain a composite waveform average onset or offset for each 24 h period and each animal. The size of heart rate drop and the maximal gradient of the rate of change of heart rate were recorded for each day of analysis. As with the baseline parameters, a rolling 3 day average was performed for each parameter prior to final analysis to allow effective interpolation of battery-life-preserving ‘off days’.
In vitro measurement of cardiac response to vagal nerve stimulation
The in vitro technique has previously been described (Choate et al. 2001). In brief, animals were killed by cervical dislocation. The atria and right vagus nerve were isolated mounted in an organ bath at 36.5°C in carbogen-bubbled mouse Ringer solution (in mmol l−1: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 0.5 Na2EDTA, 1.2 KH2PO3, 25 NaHCO3, 11 glucose and 1.75 CaCl2, pH 7.4). Atrial rate was measured using a pressure transducer attached to the left atrial appendage. The right vagus nerve was directly stimulated using a silver bipolar electrode at 3 or 5 Hz for 30 s each (10 V, 1 ms pulse width) and the change in atrial rate determined. These experiments were performed on singly housed exercise-trained mice and sedentary littermates provided with a non-rotating wheel.
Statistics
Results are expressed as the means ± SEM. Data were compared with Student’s paired t test unless otherwise stated, with P values modified to account for multiple tests performed using the Bonferroni correction. Where appropriate, different time periods were analysed separately.
Results
Voluntary running in telemetered mice
All implanted mice ran freely on the running track provided. Telemetered mice showed a good capacity for voluntary exercise, achieving on average over 2 h of running per night at a cruising speed of more than 45 m min−1. Telemetered animals also showed a characteristic training response (Allen et al. 2001; De Bono et al. 2006); time spent running peaked around day 7 before reaching a plateau and gradually declining, whilst cruising speed peaked around day 15 before plateauing thereafter.
Haemodynamic consequences of acute exercise
In order to determine the differential haemodynamic response to increasing intensities of exercise, we analysed beat-to-beat changes in blood pressure and heart rate during spontaneous wheel running, cage activity and at rest. The typical 24 h haemodynamic profile was multimodal, with each peak representing specific blood pressure and heart rate combinations (Fig. 1). Rest, cage activity and wheel running were associated with distinct blood pressure and heart rate profiles (ANOVA for repeated measures, P < 0.001 for both heart rate and blood pressure).
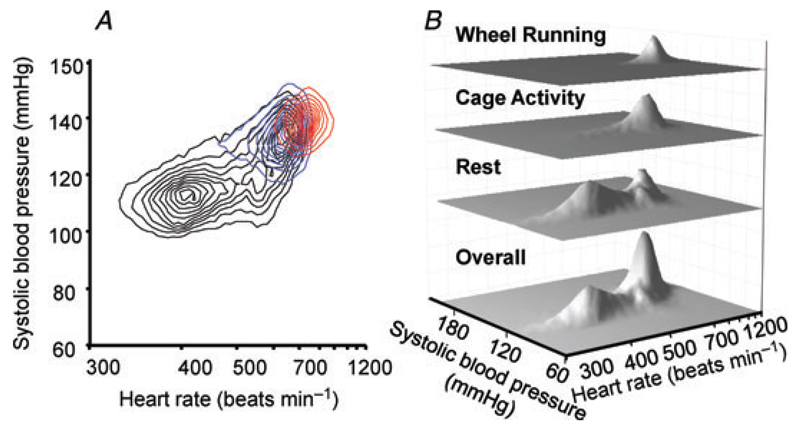
Figure 1. Typical example of distribution for systolic blood pressure and heart rate over a 24 h period in a single mouse.
A, contour plot showing haemodynamic distribution at rest (black contours), during cage activity (blue) and during wheel running (red). B, three-dimensional interval histogram showing distribution of haemodynamic parameters overall, at rest, during cage activity and during wheel running. The lower rest mode occurs at 115 mmHg and 400 beats min−1 in this example, the cage activity mode at 135 mmHg and 650 beats min−1 and the wheel running mode at 140 mmHg and 730 beats min−1. There is also a second resting peak, coinciding exactly with the cage activity peak.
Wheel running was associated with a single haemodynamic peak at highest blood pressure and heart rate (e.g. 140 mmHg and 730 beats min−1 in Fig. 1). Cage activity also coincided with a single haemodynamic mode (e.g. 135 mmHg and 650 beats min−1 in Fig. 1). Heart rate during wheel running was significantly higher than during cage activity (Fig. 2), whereas blood pressure was only marginally increased (Fig. 3). Rest was associated with a more complex haemodynamic profile, usually incorporating more than one peak. The most prominent peak was at a considerably lower blood pressure and heart rate than activity (e.g. 115 mmHg and 400 beats min−1 in Fig. 1; P < 0.001 heart rate in Fig. 2; P < 0.001 systolic blood pressure in Fig. 3). A second peak, at higher heart rate and blood pressure, coincided exactly with the haemodynamic mode for activity (e.g. 135 mmHg and 650 beats min−1 in Fig. 1), suggesting that during these periods of physical immobility the mice remained alert, with a haemodynamic profile consistent with activity.
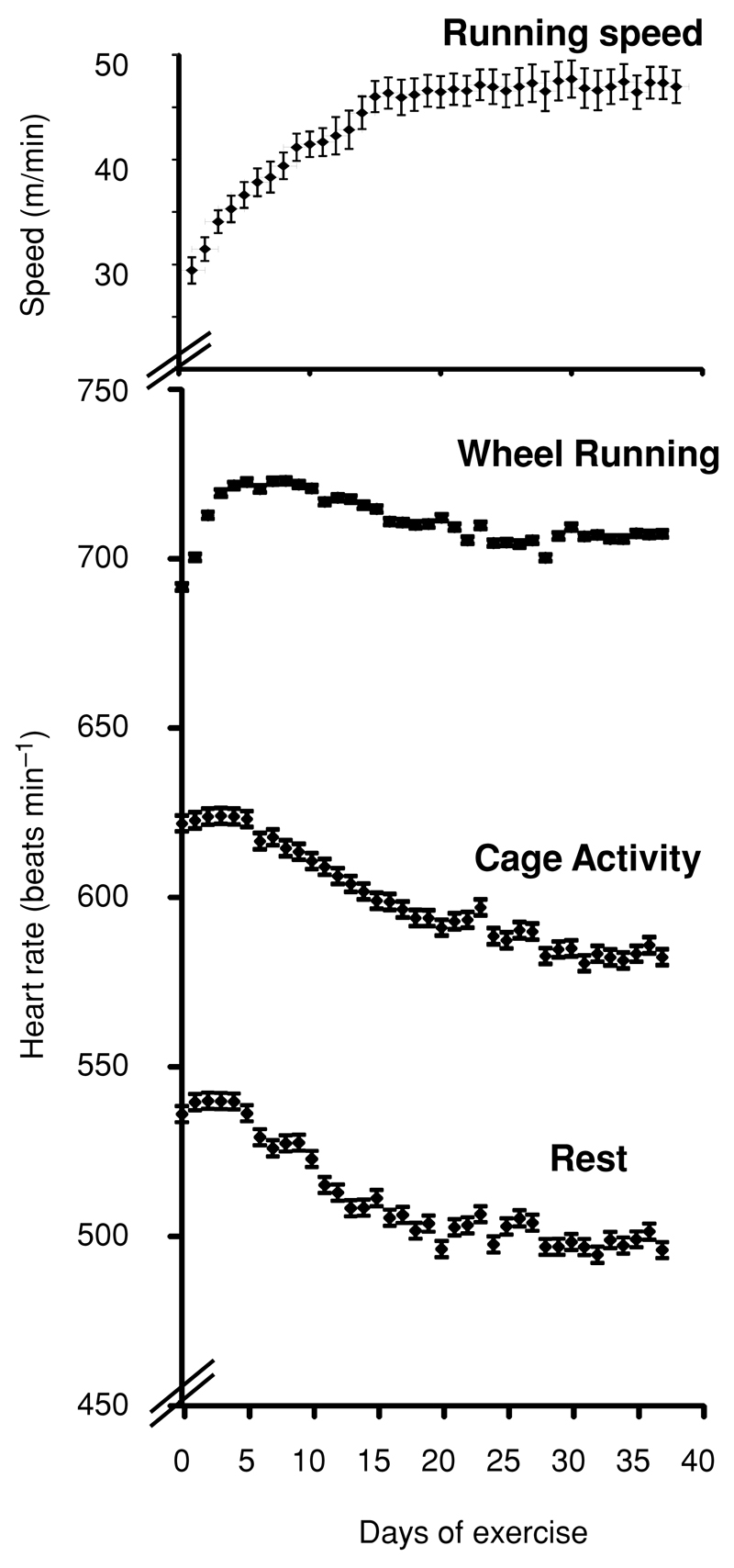
Figure 2. Effect of chronic voluntary exercise training on mean heart rate during different states of activity.
Values are means ± SEM; n = 14. There is a significant fall in resting heart rate (P < 0.01 for days 1–7 versus days 20–38) and heart rate during cage activity (P < 0.05 for days 1–7 versus days 20–38), but a rise in heart rate during wheel running during the early stages of training. The effect of exercise training on cruising speed is shown on the same time axis for comparison.
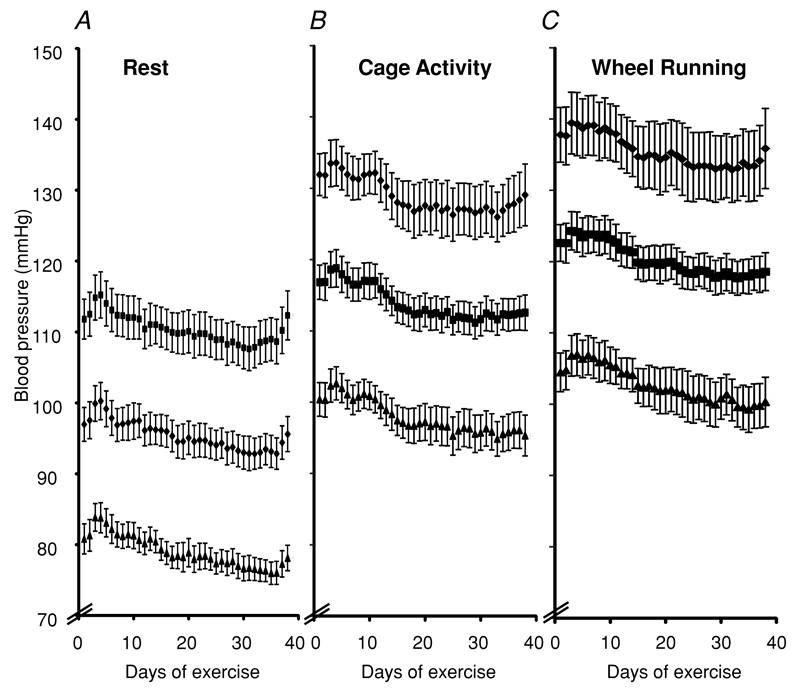
Figure 3. Effect of chronic voluntary exercise training on blood pressure during different states of activity.
Systolic, diamonds; mean, squares; and diastolic, triangles. Blood pressure is shown at rest (A), during cage activity (B) and during wheel running (C). n = 14. There is a gradual decline in blood pressure with exercise training, which only reaches significance for the resting mode (P < 0.05 for days 1–7 versus days 31–38).
Change in exercise state from rest to cage activity led to an increase in both blood pressure and heart rate, whilst the transition from cage activity to wheel running led predominantly to a rise in heart rate (Fig. 4). Indeed, the rise in blood pressure from cage activity to wheel running was very small (mean 5 mmHg), with a much greater increase occurring at the initiation of lower intensity cage activity from rest (mean 20 mmHg; Fig. 4). There was no significant increase in pulse pressure with either cage activity or wheel running (first 5 days: rest, 31.1 mmHg ± 1.53; active, 31.2 mmHg ± 1.66; and exercise, 36.3 mmHg ± 2.48).
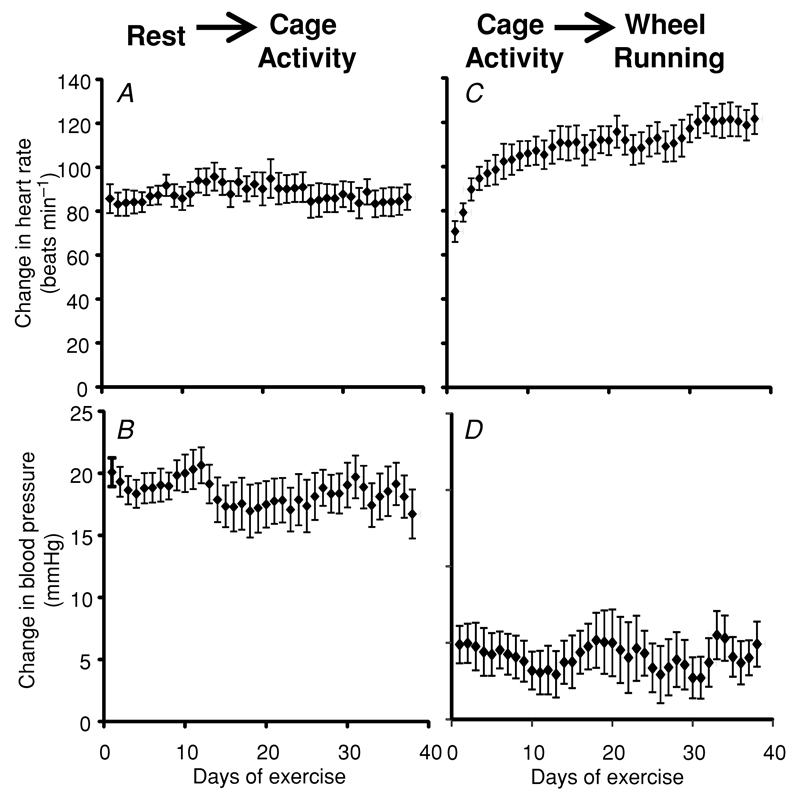
Figure 4. Effect of chronic voluntary exercise training on the average increment in mean heart rate and systolic blood pressure between different activity states.
Values (means ± SEM) are shown between rest and cage activity (A and B) and from cage activity to exercise (C and D). n = 14. Both heart rate and blood pressure markedly increase between rest and cage activity, but the predominant additional effect of wheel running is an increase in heart rate with only a small rise in blood pressure. With chronic exercise training, there is a significant increase in the heart increment rate from cage activity to wheel running (P < 0.05 for days 1–7 versus days 20–38). Most of this increase occurs between days 1 and 20 of voluntary running. In contrast, there is no significant effect of exercise training on the increase in blood pressure between any of the activity states.
Effect of chronic exercise training on haemodynamic parameters
We next evaluated the effects of chronic exercise training on blood pressure and heart rate during rest, cage activity and wheel running. As exercise training progressed, there was a significant fall in resting heart rate. The mean heart rate fell by 37 ± 7 beats min−1, and reached a minimum by day 20 (P < 0.01 for days 1–7 versus days 20–38), coinciding exactly with the time taken for the cruising speed of wheel running to reach a stable maximum (Fig. 2). Resting mean heart rate then reached a plateau thereafter, although with some variability. Heart rate during cage activity also declined with exercise training (Fig. 2; from 622 to 586 beats min−1, P < 0.01, for days 1–7 versus days 20–38). In contrast to the reduction in heart rates during rest and activity, heart rate during wheel running initially rose rapidly in the first few days of wheel running before declining to reach a stable plateau.
The overall consequence of the changes in heart rate with exercise training was thus a significant increase in the heart rate increment from cage activity to wheel running (average increase from active to exercise, 90 beats min−1 for days 1–7 versus 116 beats min−1 for days 20–38, P < 0.01; Fig. 4), but with little change in the increment from rest to activity.
In contrast to the changes seen in heart rate, chronic exercise training induced only a small fall in blood pressure. Following an initial rise in blood pressure during the first 2 days of exercise, there was then a small but steady trend towards a blood pressure decline in all activity states. This only reached significance for resting systolic blood pressure (P < 0.05 for days 3–7 versus days 31–38), with a downward trend in active and wheel running blood pressure modes (Fig. 3). This fall in blood pressure did not appear to plateau, but continued at least until the end of the training period in these experiments. There was no significant change in the increment in blood pressure from rest to activity or from activity to wheel running with chronic exercise training and no significant change in pulse pressure with exercise training.
Heart rate onset and recovery during voluntary wheel exercise
We next aimed to quantify the nature of the acute changes in heart rate that occurred at the onset of and recovery from episodes of exercise, in each 24 h period. Onset of wheel running was associated with a rapid rise in heart rate (Fig. 5A). During exercise training, the magnitude of the increase in heart rate at onset of exercise did not change significantly. Chronic exercise training, however, was associated with a marked increase in the peak rate of change in heart rate at onset of exercise (Fig. 5B; 6.2 beats min−1 s−1 for days 1–7 versus 8.4 beats min−1 s−1 for days 20–35, P < 0.01).
Figure 5. Changes in heart rate at the onset and offset of voluntary wheel running.
A, a typical example of the rapid rise in heart rate at the onset of wheel running. B, effect of chronic voluntary exercise training on the mean peak rate of heart rate increase at the onset of wheel exercise (means ± SEM, n = 6). This shows a significant rise in the peak gradient of heart rate onset (P < 0.01), with most of the increase occurring between days 7 and 15 of exercise. C, a typical example of the rapid fall in heart rate at the cessation of wheel running. D, effect of chronic voluntary exercise training on the mean peak rate of heart rate recovery at the cessation of wheel exercise (means ± SEM, n = 7). This shows a significant rise in the peak gradient of heart rate recovery (P < 0.01), reaching a peak at day 20 and plateauing thereafter.
At the end of individual bouts of voluntary wheel running, heart rate fell rapidly (Fig. 5C). Exercise training significantly increased the peak rate of heart rate recovery, as measured by the maximal gradient of the rate of change of heart rate (Fig. 5D; 4.5 beats min−1 s−1 for days 1–7 versus 5.9 beats min−1 s−1 for days 20–35, P < 0.01). The rate of heart rate recovery increased to a maximum by day 20 of exercise training and remained stable thereafter.
In vitro confirmation of vagal training in response to chronic voluntary wheel running
Confirmation of a training effect on cardiac vagal function was obtained by in vitro measurements of cardiac vagal responsiveness, on non-telemetered exercising and sedentary littermates. Chronic exercise training was associated with a marked increase in the response to direct vagal nerve stimulation (Fig. 6).
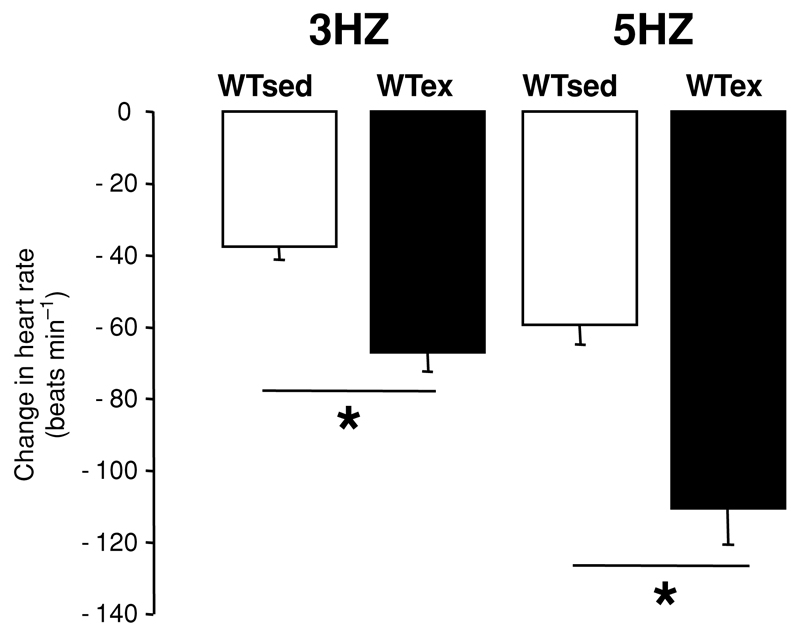
Figure 6. Response to right vagal nerve stimulation in vitro on atrial rate in mice following 4 weeks of voluntary exercise training (WTex) and in sedentary control mice (WTsed).
The effect of direct stimulations at 3 and 5 Hz is shown. n = 10 per group. There was a significant increase in the response to vagal stimulation in exercised animals. Values are means ± SEM. * P < 0.001.
Discussion
In this study we report, for the first time, the use of radiotelemetry in mice to measure real-time changes in blood pressure and heart rate during voluntary wheel running. No previous study has reported the accurate temporal alignment of running wheel rotations with a radiotelemetric haemodynamic recording. This enables precise phenotyping of murine haemodynamic changes during different activity states, during the onset and recovery from exercise bouts and during voluntary exercise training without the stressors associated with tethering or forced treadmill exercise.
Using this approach has allowed us to phenotype and quantify important new aspects of the autonomic response to both acute and chronic exercise in mouse models. We have demonstrated first, that rest, cage activity and wheel running are associated with distinct haemodynamic profiles in mice; second, that the transition from rest to cage activity is associated with a marked increase in both heart rate and blood pressure, whilst the predominant haemodynamic effect of acute wheel running, in contrast, is a large and rapid rise in heart rate with only a very small increase in blood pressure; third, that chronic exercise training is associated with a decline in resting heart rate but an initial rise in heart rate during wheel running; and fourth, that the peak rate of change of heart rate at the onset of and recovery from individual exercise bouts, but not the magnitude of that change, significantly increases with chronic wheel running. This new phenotyping tool will allow detailed in vivo analysis in genetic mouse models of the mechanisms underlying the autonomic control of cardiac function.
The use of mouse models allows investigation of the effects of genetic modifications in vivo. In contrast to man, however, mice have a high resting heart rate and limited parasympathetic tone at baseline. Exercise provides a means to enhance vagal function and investigate the dynamic process of vagal training in vivo. The published data describing murine autonomic responses to exercise are limited (Kaplan et al. 1994; Desai et al. 1997; Rohrer et al. 1998; Masuki et al. 2003; De Angelis et al. 2004; Gu et al. 2004). Most murine exercise haemodynamic studies to date have used a carotid-implanted fluid-filled catheter tunnelled to the back, exteriorized and attached to a distant pressure transducer. This approach is subject to a number of technical challenges and stresses to the animal (Van Vliet et al. 2000). In addition, difficulties in maintaining catheter patency mean that studies are typically of short duration and are often performed a relatively short time after surgery (Desai et al. 1997; Rohrer et al. 1998; Masuki et al. 2003), despite evidence to suggest that the normal diurnal variations in haemodynamics take 7–10 days to recover after surgery (Whitesall et al. 2004).
Radiotelemetry allows long-term haemodynamic monitoring of voluntary wheel running in normal cage conditions. Improved battery technology has allowed for the production of a new generation of smaller murine blood pressure telemeter implants (PAC10 DSI Transoma Medical Inc.). Combined use of this evolving technology with a novel approach to data analysis (Spike2; Cambridge Electronic Design Inc.) to precisely align in time the beat-to-beat blood pressure data with wheel rotation data has allowed us not only to investigate the acute heart rate response and blood pressure to both lower intensity cage activity or higher intensity voluntary wheel running, but also to examine the effect of chronic exercise training on autonomic control of the cardiovascular system.
It has previously been established that haemodynamic parameters in mice are not normally distributed. Instead, there are preferred modal peaks of blood pressure and heart rate, the lowest of which occurs largely during periods of rest and during daylight hours and the higher of which coincides with periods of greater activity and at night (Janssen et al. 2000; Van Vliet et al. 2003). Our data confirm that activity was associated with a distinct haemodynamic profile, with increased blood pressure and heart rate compared with rest. Furthermore, higher intensity wheel running led to further changes in the haemodynamic profile, with additional increases in heart rate but only a slightly higher blood pressure. Indeed, in trained animals 85% of the increase in the rate–pressure product occurring as exercise intensity increased from cage activity to wheel running was due to the rise in heart rate, whereas between rest and cage activity only 62% was attributable to heart rate, with 38% due to the blood pressure increase. This is in contrast to humans, where there is a graded increase in both heart rate and blood pressure with incremental exercise (Sharman et al. 2005). The lack of further response of blood pressure to higher intensities of exercise may in part be explained by the murine heart force–frequency relationship. At lower heart rates, increases in heart rate are associated with an increase in myocardial contractility, but at higher heart rates (>600 beats min−1), this relationship plateaus (Georgakopoulos & Kass, 2001). In the human heart, increases in heart rate are accompanied by increases in contractility throughout the physiological range.
There are few previous reports of the effects of chronic exercise training on heart rate and blood pressure in mice (Kaplan et al. 1994; De Angelis et al. 2004; Gu, 2004; Falcao et al. 2010; Schuler et al. 2010). Our data show that voluntary exercise training has distinct effects on the haemodynamic profiles associated with different activity states. The major effects were on heart rate. Heart rate both at rest and during cage activity fell with regular exercise, but the effect was more marked for resting heart rate. These changes were complete by day 20 of voluntary wheel running, a time course which coincided exactly with the number of days of training required to reach the maximal cruising speed (the modal running speed). This suggests that cruising speed may be a better running measure of physiological training during voluntary wheel running than more traditional running parameters, such as distance run or time spent exercising, which show a more rapid increase (Allen et al. 2001; De Bono et al. 2006). The overall effect of exercise training in C57BL/6J mice was therefore to maximize the heart rate increase, both from rest to cage activity and from cage activity to wheel running. This may lead to an increase in cardiac output in response to different activity states, allowing for the improvement in running performance reflected by the parallel rise in cruising speed.
The effect of chronic exercise training on blood pressure was surprisingly minimal, with only a small decrease in blood pressure parameters whether at rest, during cage activity or during wheel running. Thus, in mice the increase in cardiac performance with chronic voluntary exercise training appears largely to be due to effects on heart rate, in contrast to humans, where both blood pressure and heart rate are important. The minimal blood pressure response to training in mice may in part be related to the small increase in blood pressure seen with acute voluntary wheel running, acting as only a modest stimulus for chronic blood pressure adaptation. Furthermore, in contrast to the changes in heart rate, which reached a clear maximum 20 days after the onset of voluntary running, the gradual decline in blood pressure appeared to continue throughout the study period. This suggests that the mechanisms involved in mediating the blood pressure response to exercise training may be distinct from those responsible for the changes described for heart rate. Further reductions in blood pressure may also be possible with more prolonged exercise. These findings are consistent with the only previous report of the haemodynamic effects of voluntary exercise in mouse models (Falcao et al. 2010). This demonstrated no change in 24 h averaged mean arterial blood pressure over 1 month. Falcao et al. (2010) did not simultaneously measure wheel rotations and telemetered haemodynamics and so do not report haemodynamic variations with different activity states or at the onset of or recovery from exercise bouts. There is also no description of the effects of exercise training on heart rate.
The acute changes at the onset and offset of exercise are closely associated with autonomic function in humans (Freeman et al. 2006). In humans, patients with a reduced heart rate response during exercise testing (‘chronotropic incompetence’) have a greater risk of coronary heart disease events and mortality (Sandvik et al. 1995; Lauer et al. 1996, 1999). Heart rate recovery following exercise is also an independent marker of cardiovascular risk in humans (Cole et al. 1999, 2000; Nishime et al. 2000; Shetler et al. 2001; Aktas et al. 2004; Jouven et al. 2005). It has not previously been possible to measure these parameters following voluntary wheel exercise in mouse models. However, our studies have now revealed that the onset of voluntary exercise in mice is accompanied by a very rapid chronotropic response. Equally, heart rate recovery following cessation of wheel exercise is also extremely rapid, taking less than 30 s (compared with humans, where recovery is of the order of minutes). Chronic exercise caused a significant increase in the peak rate of both the rise in heart rate at the onset of exercise and heart rate recovery at the end of exercise. This increase occurred between days 7 and 15 of exercise training.
The changes in both resting heart rate and heart rate recovery are consistent with enhanced parasympathetic function with exercise training. This is further supported by the in vitro data, confirming a significant upregulation of vagal responsiveness in exercise-trained animals compared with sedentary littermates.
One limitation of this study is that haemodynamic measurements are not presented prior to running wheel insertion. It is possible that the stress associated with this environmental change may have contributed to some early haemodynamic changes. We have previously shown that a similar stress leads to an elevation in heart rate for around 60 min (data not shown) and decided to focus telemeter battery life on recordings during the exercise training period. We do not feel that this effect would substantially confound the data presented. Another potential limitation is the lack of a sedentary control group. There are, however, established data to demonstrate that apart from diurnal varation, there is no systematic change in haemodynamic parameters in the time period of the study presented (Whitesall et al. 2004). We therefore elected to focus telemetry resources on exercising animals.
It is clear that regular physical activity has multiple benefits in humans, both in reducing cardiovascular risk and in improving prognosis in patients with cardiovascular disease. Monitoring heart rate and blood pressure responses to exercise is now a routine part of the clinical assessment of patients with cardiovascular disease. Mouse models are increasingly allowing the molecular genetic mechanisms underlying these beneficial effects to be investigated. Radiotelemetry of the dynamic effects of exercise on haemodynamic parameters in genetic mouse models, coupled with the long-term haemodynamic effects of exercise training, may provide novel insight into the central role of haemodynamic regulation in cardiovascular health and disease.
Acknowledgements
We thank Chris Hirst for technical support and Steven Clifford (Cambridge Electronic Design Ltd, Cambridge, UK) for programming assistance. This work was supported by a Medical Research Council Grant to D.J.P. and K.M.C. D.A. is a Wellcome Trust Cardiovascular Research Initiative Research Fellow. J.P.De B. is a Bristol Myers Squibb Cardiovascular Research Fellow.
References
- Aktas MK, Ozduran V, Pothier CE, Lang R, Lauer MS. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA. 2004;292:1462–1468. doi: 10.1001/jama.292.12.1462. [DOI] [PubMed] [Google Scholar]
- Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- Balady GJ, Larson MG, Vasan RS, Leip EP, O’Donnell CJ, Levy D. Usefulness of exercise testing in the prediction of coronary disease risk among asymptomatic persons as a function of the Framingham risk score. Circulation. 2004;110:1920–1925. doi: 10.1161/01.CIR.0000143226.40607.71. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–1182. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- Bernstein D. Exercise assessment of transgenic models of human cardiovascular disease. Physiol Genomics. 2003;13:217–226. doi: 10.1152/physiolgenomics.00188.2002. [DOI] [PubMed] [Google Scholar]
- Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- Choate JK, Danson EJ, Morris JF, Paterson DJ. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am J Physiol Heart Circ Physiol. 2001;281:H2310–H2317. doi: 10.1152/ajpheart.2001.281.6.H2310. [DOI] [PubMed] [Google Scholar]
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- Davisson RL, Kim HS, Krege JH, Lager DJ, Smithies O, Sigmund CD. Complementation of reduced survival, hypotension, and renal abnormalities in angiotensinogen-deficient mice by the human renin and human angiotensinogen genes. J Clin Invest. 1997;99:1258–1264. doi: 10.1172/JCI119283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis K, Wichi RB, Jesus WR, Moreira ED, Morris M, Krieger EM, Irigoyen MC. Exercise training changes autonomic cardiovascular balance in mice. J Appl Physiol. 2004;96:2174–2178. doi: 10.1152/japplphysiol.00870.2003. [DOI] [PubMed] [Google Scholar]
- De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol. 2006;290:R926–R934. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol Heart Circ Physiol. 1997;272:H1053–H1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- Dorn J, Naughton J, Imamura D, Trevisan M. Results of a multicenter randomized clinical trial of exercise and long-term survival in myocardial infarction patients: the National Exercise and Heart Disease Project (NEHDP) Circulation. 1999;100:1764–1769. doi: 10.1161/01.cir.100.17.1764. [DOI] [PubMed] [Google Scholar]
- Erikssen G, Bodegard J, Bjørnholt JV, Liestøl K, Thelle DS, Erikssen J. Exercise testing of healthy men in a new perspective: from diagnosis to prognosis. Eur Heart J. 2004;25:978–986. doi: 10.1016/j.ehj.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Falcao S, Bisotto S, Michel C, Lacasse AA, Vaillancourt C, Gutkowska J, Lavoie JL. Exercise training can attenuate preeclampsia-like features in an animal model. J Hypertens. 2010;28:2446–2453. doi: 10.1097/HJH.0b013e32833e97d0. [DOI] [PubMed] [Google Scholar]
- Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. 2006;48:342–362. doi: 10.1016/j.pcad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos D, Kass D. Minimal force–frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol. 2001;534:535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122:1221–1238. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- Gu JG, Giovani G, Granger JP, Kodali M, Fortepiani L, Hall JE. Exercise training reduces blood pressure in ldlr gene-knockout (–/–) mice. Hypertension. 2004;44:507–508. [Google Scholar]
- Janssen BJ, Leenders PJ, Smits JF. Short-term and long-term blood pressure and heart rate variability in the mouse. Am J Physiol Regul Integr Comp Physiol. 2000;278:R215–R225. doi: 10.1152/ajpregu.2000.278.1.R215. [DOI] [PubMed] [Google Scholar]
- Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001;(1):CD001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958. doi: 10.1056/NEJMoa043012. [DOI] [PubMed] [Google Scholar]
- Kaplan ML, Cheslow Y, Vikstrom K, Malhotra A, Geenen DL, Nakouzi A, Leinwand LA, Buttrick PM. Cardiac adaptations to chronic exercise in mice. Am J Physiol Heart Circ Physiol. 1994;267:H1167–H1173. doi: 10.1152/ajpheart.1994.267.3.H1167. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–843. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Maass AH, Luckey SW, Stauffer BL, Olson EN, Leinwand LA. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287:H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25:1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH. Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA. 1999;281:524–529. doi: 10.1001/jama.281.6.524. [DOI] [PubMed] [Google Scholar]
- Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–1526. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol. 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- Masuki S, Takeoka M, Taniguchi S, Yokoyama M, Nose H. Impaired arterial pressure regulation during exercise due to enhanced muscular vasodilatation in calponin knockout mice. J Physiol. 2003;553:203–212. doi: 10.1113/jphysiol.2003.047803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson DL. Long-term measurement of arterial blood pressure in conscious mice. Am J Physiol Regul Integr Comp Physiol. 1998;274:R564–R570. doi: 10.1152/ajpregu.1998.274.2.R564. [DOI] [PubMed] [Google Scholar]
- Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol. 2000;88:1537–1544. doi: 10.1152/jappl.2000.88.5.1537. [DOI] [PubMed] [Google Scholar]
- Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.37938.645220.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer DK, Schauble EH, Desai KH, Kobilka BK, Bernstein D. Alterations in dynamic heart rate control in the β1-adrenergic receptor knockout mouse. Am J Physiol Heart Circ Physiol. 1998;274:H1184–H1193. doi: 10.1152/ajpheart.1998.274.4.H1184. [DOI] [PubMed] [Google Scholar]
- Sandvik L, Erikssen J, Ellestad M, Erikssen G, Thaulow E, Mundal R, Rodahl K. Heart rate increase and maximal heart rate during exercise as predictors of cardiovascular mortality: a 16-year follow-up study of 1960 healthy men. Coron Artery Dis. 1995;6:667–679. doi: 10.1097/00019501-199508000-00012. [DOI] [PubMed] [Google Scholar]
- Schuler B, Arras M, Keller S, Rettich A, Lundby C, Vogel J, Gassmann M. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc Natl Acad Sci USA. 2010;107:419–423. doi: 10.1073/pnas.0912924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman JE, McEniery CM, Campbell RI, Coombes JS, Wilkinson IB, Cockcroft JR. The effect of exercise on large artery haemodynamics in healthy young men. Eur J Clin Invest. 2005;35:738–744. doi: 10.1111/j.1365-2362.2005.01578.x. [DOI] [PubMed] [Google Scholar]
- Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behav Genet. 1998;28:227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skidmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–692. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- Van Vliet BN, Chafe LL, Antic V, Schnyder-Candrian S, Montani JP. Direct and indirect methods used to study arterial blood pressure. J Pharmacol Toxicol Methods. 2000;44:361–373. doi: 10.1016/s1056-8719(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol. 2003;549:313–325. doi: 10.1113/jphysiol.2003.041897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]