Scheme 1. (a) Proposed Oxidative Dimerization To Form Lomaiviticins A and B (1 and 2), (b) Elimination–Aromatization Pathway, and (c) Syntheses of the Diazofluorenes 18 and 19a.
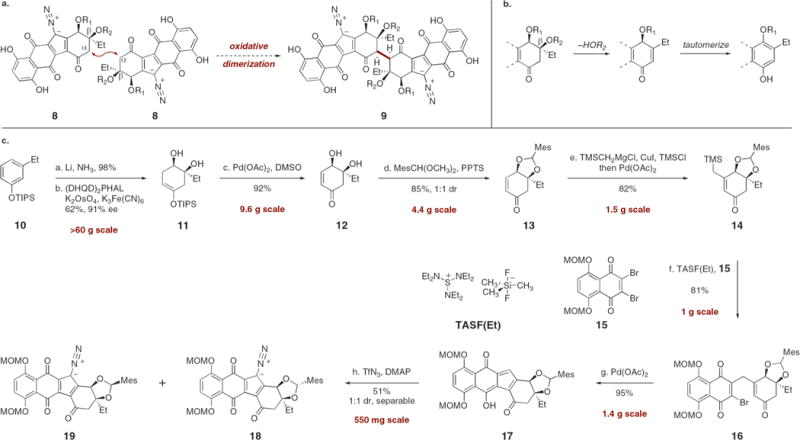
aConditions: (a) Li, NH3, t-BuOH, THF, −78 → −33 → −78 °C, 98%. (b) (DHQD)2PHAL, K2OsO4, CH3SO2NH2, t-BuOCH3–t-BuOH-H2O, −5 °C, 62%, 91% ee. (c) Pd(OAc)2, O2, DMSO, 24 °C, 92%. (d) MesCH(OCH3)2, PPTS, CH3CN, 24 → 50 °C, 85%. (e) TMSCH2MgCl, CuI, HMPA, Et3N, TMSCl, THF, −30 → −60 → −78 °C; Pd(OAc)2, CH3CN, 24 °C, 82%. (f) TASF(Et), CH2Cl2, −78 °C, 81%. (g) Pd(OAc)2, polymer-supported PPh3, Ag2CO3, toluene, 80 °C, 95%. (h) TfN3, DMAP, CH3CN, −20 °C, 51%.
