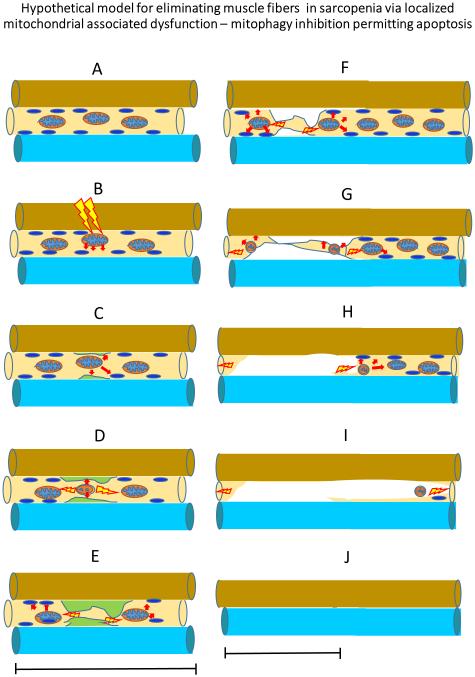
Figure 2. Hypothetical model for eliminating muscle fibers in sarcopenia via localized mitochondrial associated dysfunction – mitophagy and apoptosis.
A. In healthy muscle, activation of mitophagy eliminates dysfunctional nuclei so that they cannot continue death signaling. B. Dysfunctional mitochondria that leak their contents to the cytosol will occur in muscle that has received a significant mitochondrial stress (e.g., ROS, inflammatory mediators etc.). C-D. This initiates the apoptotic signaling cascades. E-F. If the dysfunctional mitochondria are not eliminated, apoptotic death signaling may be activated to eliminate myonuclei and this may concurrently or independently result in wide-spread activation of autophagy and the ubiquitin ligase pathway and also, trigger the necrosis signaling pathway to remove muscle proteins, mitochondria and nuclei within the domain of the initial dysfunctional mitochondria (G-H). I-J. The extent of dysfunctional mitochondrial will extend along the mitochondrial reticular network and affect the function of other mitochondria near the dysfunctional mitochondrial. The wider accumulation of dysfunctional mitochondria will perpetuate signaling for apoptosis, which will remove nuclei from a larger area. The greater nuclear loss will be followed by elevated proteasome signaling to eliminate contractile and non-contractile tissue in the fiber segment that is associated with dysfunctional mitochondria and apoptotic signaling. This cellular removal will result in eventual elimination of the portion of the fiber in the area of the dysfunctional mitochondrial and potentially the entire fiber. We further hypothesize that the initiation of the disassembly and removal of the fiber could be blocked if the dysfunctional mitochondria which initiate the process, are removed or the damage to mitochondria reversed (e.g., via exercise and nutritional interventions) and if irreparable damaged mitochondria are replaced by healthy mitochondria.