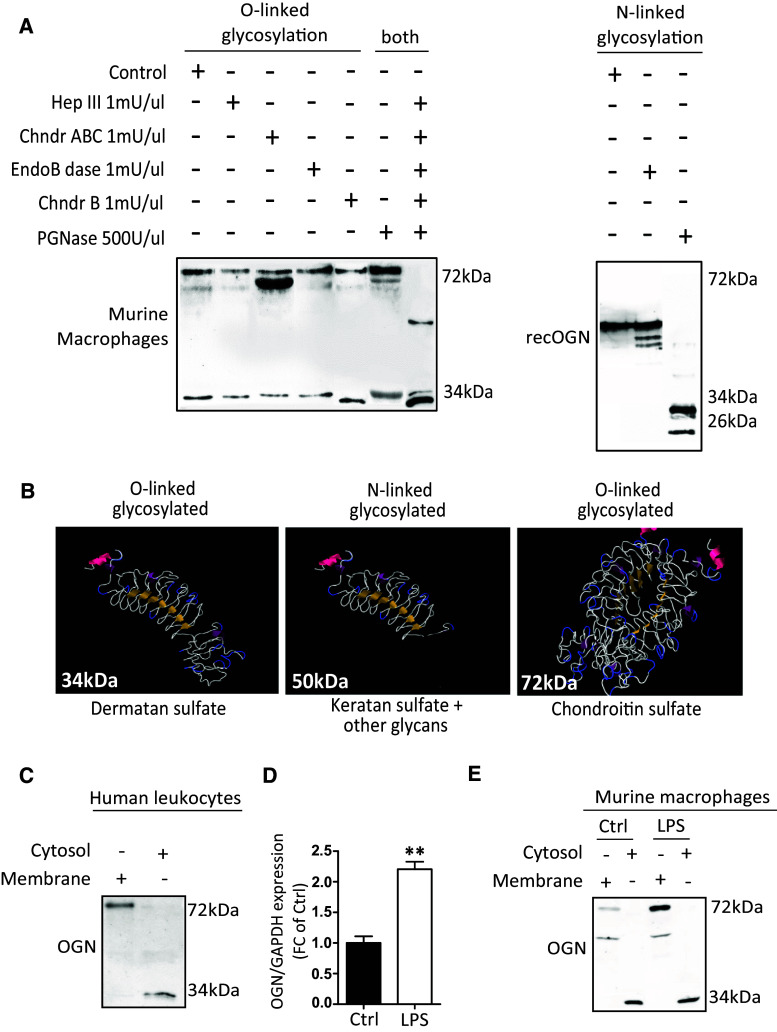
Fig. 4.
Glycosylation of the 32-kDa OGN core protein results in the production of different protein variants. a Enzyme treatment of macrophage protein lysates revealed the differential presence of glycans and glycosaminoglycans; treatment with chondroitinase ABC reduced the size of the 72-kDa protein variant, indicating that chondroitin and dermatan sulfate is attached, whereas treatment with chondroitinase B only slightly reduced the size of the 34-kDa variant, indicating that it only has dermatan sulfate attached. The simultaneous addition of all of the enzymes reduced the protein glycosylation of both the 34-kDa and 72-kDa variants entirely. Treatment of the 50-kDa OGN variant with PGNase reduced the size detected on western blot significantly, indicating that it is N-linked glycosylated. b Predicted structures of the OGN variants and their respective glycosylations. c Cell fractionation of fresh human buffy coat lysates revealed the presence of the 72-kDa OGN variant in the cell membrane, whereas the 34-kDa variant was found in the cytosol. d, e In mouse bone marrow-derived macrophages, LPS stimulation increased the expression of OGN in the cell membrane. All experiments were repeated at least twice