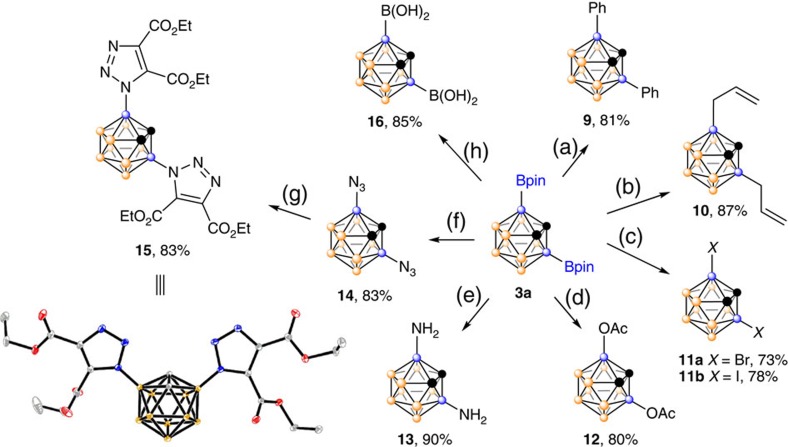
Figure 3. Chemical transformations of 3a.
Reaction conditions: (a) PhBr (3 equiv.), Pd(PPh3)4 (20 mol%), Cs2CO3 (3 equiv.), cyclohexane, 150 °C (bath), 8 h. (b) Allyl chloride (6 equiv.), Pd(dba)2 (20 mol%), Cs2CO3 (3 equiv.), toluene, room temperature, 24 h. (c) PhX (3 equiv.), Pd(PPh3)4 (10 mol%), tBuOK (3 equiv.), THF, 80 °C, 24 h. (d) Cu(OAc)2 (6 equiv.), KF (6 equiv.), CH3CN, 80 °C, 12 h, under 1 atm of O2. (e) MeONHLi, THF, 80 °C, 8 h. (f) TMSN3 (2.4 equiv.), CuCl (2.1 equiv.), KF (2.4 equiv.), THF, 60 °C, 24 h. (g) Diethyl acetylenedicarboxylate (2.4 equiv.), toluene, 95 °C. (h) 1) DEA (diethanolamine, 2.5 equiv.), Et2O, room temperature, 18 h, 2) HCl aq. (0.5 M, excess).