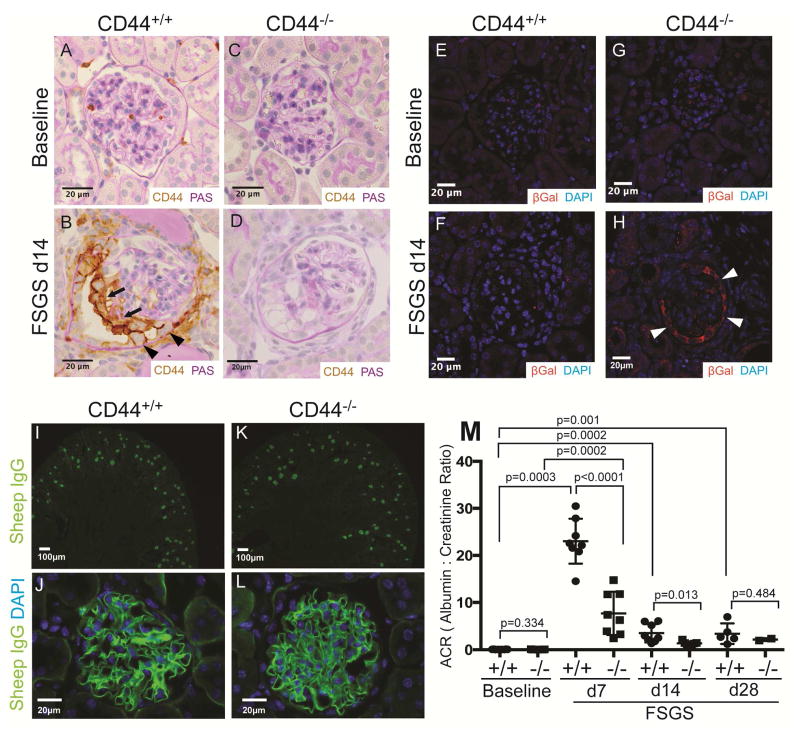
Figure 1. FSGS in CD44+/+ and CD44−/− mice.
(A–D) CD44 and PAS co-staining. (A) CD44 staining is not detected in parietal epithelial cells (PECs) in CD44+/+ mice at baseline. (B) In CD44+/+ mice at day (d) 14 of FSGS, CD44 staining (brown) is detected in cells lining Bowman’s capsule (arrow heads), and also along the outer aspect of the glomerular tuft (thin arrows). (C) CD44 staining was not detected in glomeruli of CD44−/− mice at baseline, nor at d14 of FSGS (D). These results show that CD44 was de novo expressed in PECs and podocytes only in CD44+/+ mice at d14 of FSGS.
(E–H) β-Galactosidase (βGal) and DAPI staining. βGal staining (red color) was used to detect LacZ that was knocked into exon 1 and intron 1 of CD44, and DAPI stained nuclei (blue color). (E) and (F) shows that βGal staining was not detected in CD44+/+ mice at baseline nor d14 FSGS respectively. (G) βGal staining was not detected in CD44−/− mice at baseline. (H) βGal staining was present in cells along Bowman’s capsule in CD44−/− mice at FSGS d14. These results confirmed that the CD44 promoter was activated in PECs in CD44 null/knockin mice with FSGS.
(I–L) Sheep IgG and DAPI staining. Because a sheep anti-podocyte antibody was used to induce FSGS, sheep IgG staining (green color) was performed to assess antibody deposition at d14 of FSGS. (I) Low power view showing sheep IgG staining in cortical glomeruli in CD44+/+ mice. (J) High power view of typical sheep IgG pattern of staining along the glomerular basement membrane in CD44+/+ mice. (K) and (L) shows that in CD44−/− mice, sheep IgG staining at low and high magnification respectively was indistinguishable from CD44+/+ mice. (M) Urinary albumin to creatinine ratio (ACR). ACR were very low, but similar in CD44+/+ (circles) and CD44−/− (squares) mice at baseline. ACR was statistically higher in CD44+/+ mice with FSGS at d7 and d14 compared to CD44−/− mice.