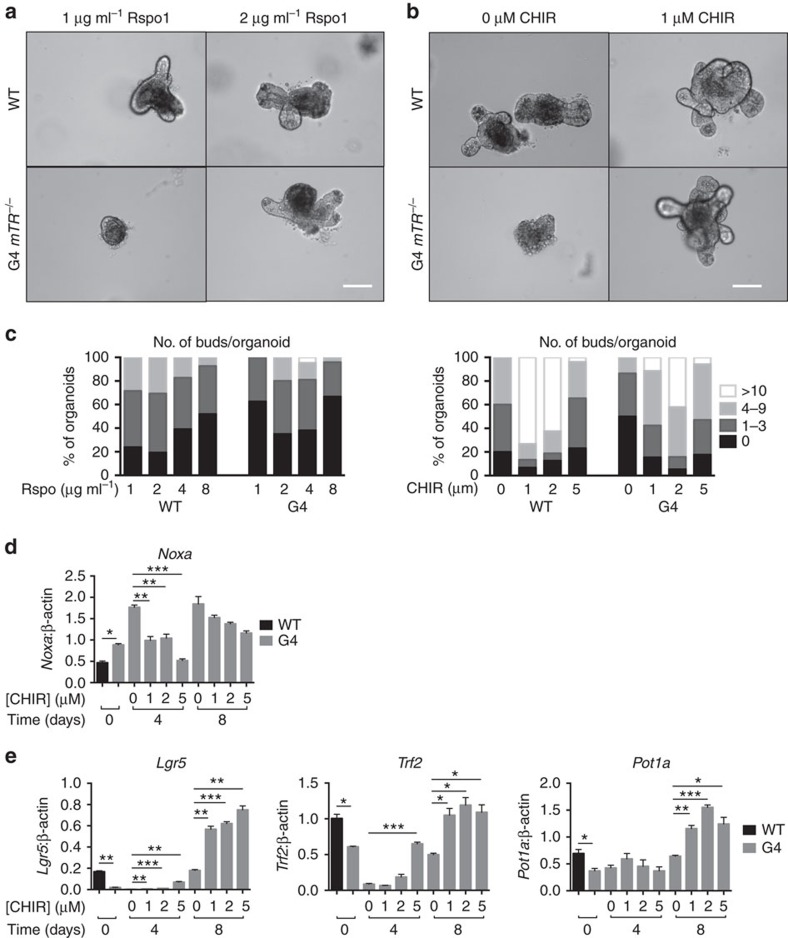
Figure 4. Wnt pathway agonists rescue survival and morphology of cultured G4 mTR−/− intestinal organoids.
(a,b) Representative micrographs showing intestinal organoids from WT and G4 mTR−/− mice cultured under standard conditions (1 μg ml−1 Rspo1) or augmented with elevated levels of (a) Rspo1 or (b) the glycogen synthase kinase 3 inhibitor CHIR99021. Under standard culture conditions, WT organoids are long-lived and appear as roughly spherical epithelium surrounding a central cavity representing the lumen, and have crypt buds radiating outward from their peripheries. G4 mTR−/− organoids were prone to degeneration and had fewer crypts under basal conditions, but could be rescued with slightly elevated Rspo1 or CHIR99021. Scale bars, 100 μm. (c) The number of buds per organoid was quantified and grouped accordingly (0, 1–3, 4–9, >10 buds per organoid), and expressed as a percentage of all organoids. Note that the well-established inhibition of crypt budding by supraphysiologic levels of Wnt pathway activity is shifted to higher doses of Rspo and CHIR in the G4 organoids, consistent with a basal Wnt pathway defect. (d) Quantitative reverse transcription PCR (qRT-PCR) measurement of Noxa transcripts, a mediator of p53-activated apoptosis, in WT and G4 mTR−/− crypts at t=0, and treated with increasing doses of CHIR99021 at days 4 or 8 (n=3); *P<0.05, **P<0.01 and ***P<0.0001. (e) Dose- and time-dependent increase of Lgr5, Tfr2 and Pot1a transcript expression as measured by qRT-PCR in WT and G4 mTR−/− crypts cultured with CHIR99021 for 4 or 8 consecutive days (n=3). For all panels, *P<0.05, **P<0.005 and ***P<0.0005. Also, note that miR34a inhibition similarly rescued organoid budding, Lgr5 and Trf2 expression (Supplementary Fig. 6). All error bars reflect s.e.m., and P values reflect unpaired two-tailed Student's t-tests.