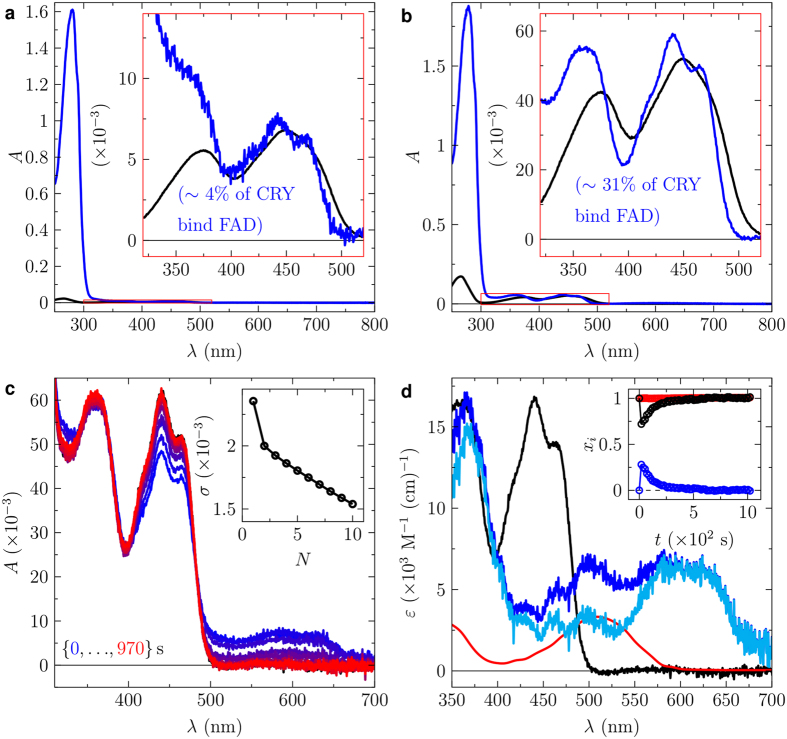
Figure 4. Recovering FAD-binding in the double mutant HsCRY2-H243R-S415N.
(a) UV-visible absorption spectrum of HsCRY2-H243R-S415N (blue line) as purified from E. coli and unbound FAD (black line). (b) UV-visible absorption spectrum of HsCRY2-H243R-S415N (blue line) after incubation in excess FAD and removal of unbound FAD by spin-filtration. Unbound FAD (black line). (c) Sequence of UV-visible absorption spectra prior and post a 1s blue-light pulse (blue to red lines). The inset shows the singular value decomposition (SVD) based principle component analysis indicating two contributing species. (d) Extracted species spectra of bound oxidized FAD isoalloxazine (black), light-induced radical pair consisting of contributions of the neutral FAD semiquinone radical and the neutral tryptophan radical (blue line), neutral tryptophan radical (red line), and neutral FAD semiquinone radical (cyan line). The inset shows the mole fractions of bound oxidized FAD and light-induced radical pair. (For details see the main text and Materials and Methods).