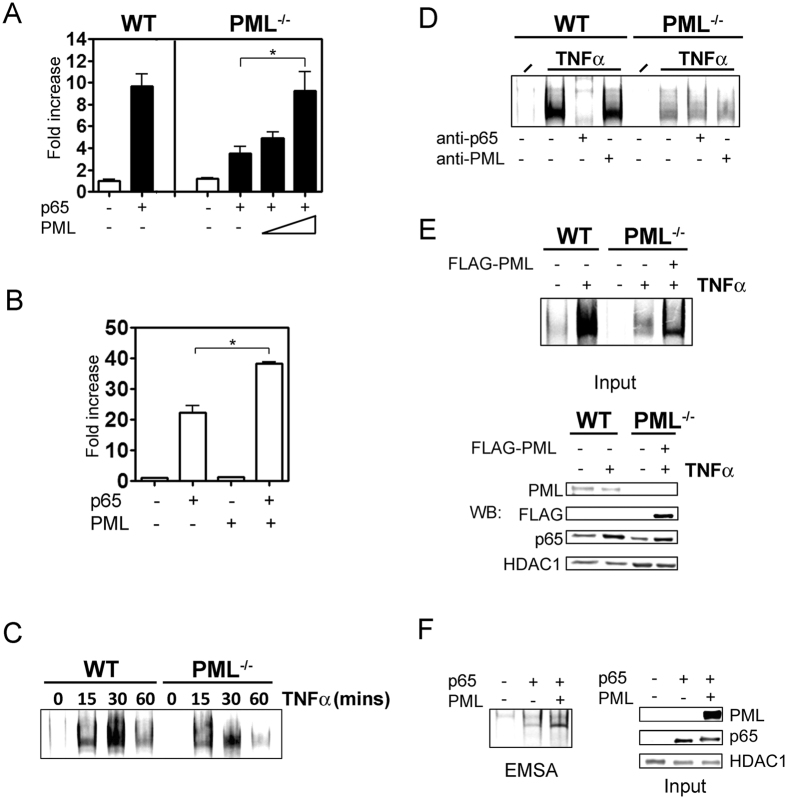
Figure 2. PML regulates NF-κB.
(A) Luciferase assay using a NF-κB consensus reporter plasmid in WT or PML−/− MEF cells co-transfected with an expression vector for p65 or increasing amounts of PML expression vector as indicated. Data presented are mean ± SEM of triplicate cultures and representative of three independent experiments. (B) Luciferase assay using NF-κB consensus reporter plasmid in HEK293T cells transfected with expression vectors for p65 and PML as indicated. Data presented are mean ± SEM of triplicate cultures and representative of three independent experiments. (C) WT and PML−/− MEFs were stimulated with TNFα (10 ng/ml) for the indicated times analysed by EMSA using oligonucleotides for the consensus NF-κB binding site. (D) WT and PML−/− MEFs were stimulated with TNFα (10 ng/ml) for one hour and analysed by EMSA using oligonucleotides for the consensus NF-κB binding site and antibodies against p65 and PML as indicated. (E) PML−/− MEFs were transfected with empty vector or an expression vector for human PML and left untreated or treated with TNFα (10 ng/ml) for one hour as indicated. Untransfected WT cells treated with TNFα (10 ng/ml for one hour) or left untreated were used as controls. Nuclear lysates used in the EMSA (input) were analysed by immunoblot using the indicated antibodies. For the detection of PML a mouse specific anti-PML antibody was used. (F) PML−/− cells were transiently transfected with PML-FLAG and p65 and analysed by EMSA using oligonucleotides for the consensus NF-κB binding site. Nuclear lysates used as input for EMSA were analysed by immunoblot using the antibodies indicated. Immunoblot for HDAC1 was used as a loading control. Data presented is representative of at least three independent experiments. Statistical significance determined by t test, p < 0.05 (*).