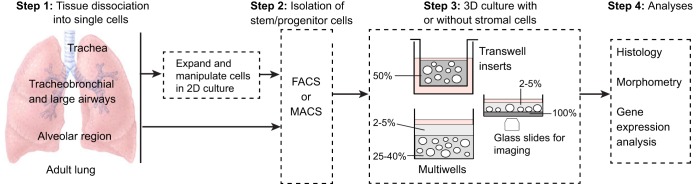
Fig. 2.
Overview of the derivation of lung organoids. Cells isolated from different regions of the adult mouse and human lung have been used for 3D culture. If intact pieces of lung are used, rather than bronchial brushings for example, the tissue is dissociated using proteases (step 1). Primary cells are isolated using FACs or MACs (magnetic bead sorting) (step 2) and can be seeded directly into Matrigel (gray, percentage indicated). In the case of basal cells, the number of undifferentiated cells can be increased by culturing them in 2D before transferring to 3D. This enables genetic manipulation and the selection and cloning of specific mutants. Methods for expanding AEC2s in 2D have not yet been reported. In Step 3, single-cell suspensions are seeded into 3D culture in inserts or multiwells, with or without mesenchymal cells (Table 1). Methods include suspending the cells in 50% Matrigel (Rock et al., 2009) or in a low concentration of Matrigel and layering this over a higher concentration into which the cells sink (Butler et al., 2016; Danahay et al., 2015; Tata et al., 2013). For live imaging, cultures can be established in glass-bottomed wells coated with a thin layer of dense Matrigel. Cells sink through the upper layer and accumulate at the interface so that they remain in the same plane for imaging (Rock et al., 2011). For histological analysis, cultures are fixed in the Matrigel. For quantification of different cell types or passaging stem cells, the Matrigel can be removed using dispase and spheres dissociated with trypsin.