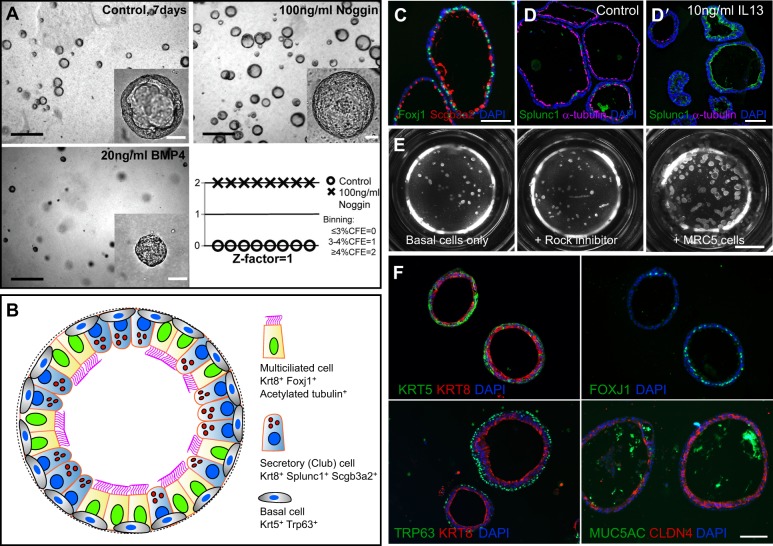
Fig. 3.
Basal cell-derived organoids. (A) Basal cell-derived organoids have been used for high- and medium-throughput screens (Danahay et al., 2015; Tadokoro et al., 2014). An example shows mouse tracheospheres after 7 days of culture, with and without 100 ng/ml noggin (a BMP inhibitor) and 20 ng/ml BMP4. The bottom right panel shows the results of scoring colony forming efficiency (CFE) in eight control wells versus eight wells with added noggin as one of the three values shown. The results were highly reproducible, giving a z factor of 1 (Zhang et al., 1999). It is important to establish such reproducibility before embarking on a large screen because conditions such as the position of a well in the tray, and changes in temperature and pH while changing the medium, can affect differentiation. (B) Schematic of a typical mouse tracheosphere after ∼14 days of culture, showing the relative position of basal versus luminal cells and markers of ciliated versus secretory cell types. (C) Section through clonal mouse tracheospheres cultured for 14 days and stained with antibody to Scgb3a2 (Club cells) and Foxj1 (ciliated cells). (D,D′) Sections of tracheospheres cultured without (D) or with (D′) 10 ng/ml IL13 and stained with DAPI and antibody to acetylated tubulin (cilia, red) and Splunc1 (Club cells, green). Note the dramatic increase in the number of secretory cells at the expense of ciliated cells in the presence of the cytokine. (E) Organoids (bronchospheres) derived from human basal cells cultured for 21 days without added factors (left), with the Rho kinase inhibitor Y-27632 (center), and with human lung fibroblasts (MRC5 line) (right). (F) Sections of human bronchospheres cultured for 21 days with MRC5 fibroblasts, stained with DAPI and markers for basal cells (KRT5, TRP63), luminal cells (KRT8, CLDN4), ciliated cells (FOXJ1) and secretory cells (MUC5AC). Scale bars: 100 µm in A insets, C,D′,F; 1 mm in A; 2 mm in E. Panel A was generated by Jason Rock; D,D′ by Tomomi Tadokoro.