ABSTRACT
Pluripotent stem cell (PSC)-derived organoids are miniature, three-dimensional human tissues generated by the application of developmental biological principles to PSCs in vitro. The approach to generate organoids uses a combination of directed differentiation, morphogenetic processes, and the intrinsically driven self-assembly of cells that mimics organogenesis in the developing embryo. The resulting organoids have remarkable cell type complexity, architecture and function similar to their in vivo counterparts. In the past five years, human PSC-derived organoids with components of all three germ layers have been generated, resulting in the establishment of a new human model system. Here, and in the accompanying poster, we provide an overview of how principles of developmental biology have been essential for generating human organoids in vitro, and how organoids are now being used as a primary research tool to investigate human developmental biology.
KEY WORDS: Pluripotent stem cells, Organoids, Human development, Directed differentiation, Patterning, Morphogenesis
Summary: This Development at a Glance article summarises how knowledge gained from developmental biology can be used to guide human in vitro organogenesis, and discusses the potential applications of this technology.
Introduction
Development of the vertebrate embryo has been the subject of intense investigation for hundreds of years. As such, many of the morphogenetic and molecular processes that regulate gastrulation, germ layer formation, patterning and morphogenesis have been well studied and found to be conserved across species. Whether this holds true for humans, however, is currently unclear, as direct functional and experimental investigation of human development has been impossible until only recently. In 1998, Thomson et al. described the first generation of embryonic stem cell (ESC) lines derived from human blastocysts (Thomson et al., 1998), which proliferate extensively and are competent to become any of the three germ layers, and these remain in use in many laboratories to this day. In 2007, Takahashi et al. described the induction of pluripotent stem cells (PSCs) – called induced PSCs (iPSCs) – from adult human fibroblasts via the transduction of four defined transcription factors: OCT3/4 (POU5F1), SOX2, KLF4 and c-MYC (Takahashi et al., 2007). The generation of iPSCs has allowed researchers and institutions to establish their own, controversy-free PSC lines and has paved the way for personalized medicine, as patient-specific iPSCs with specific and unique mutations can be generated, studied, and corrected using CRISPR/Cas9 editing (Hockemeyer and Jaenisch, 2016). Both these PSC types – iPSCs and ESCs – share an unlimited proliferative ability and the developmental potential to generate all three germ layers. As such, they form the foundation of a model system for the study of human developmental biology.
The remarkable ability of PSCs to differentiate into all cell types of the body is also one of the greatest challenges for scientists; specifically, how to control the differentiation of PSCs into distinct cell and tissue types. In the 1990s, several groups began to manipulate murine ESCs in vitro using factors known to influence gastrulation and patterning in model organisms in vivo (Desbaillets et al., 2000; Eiraku et al., 2011, 2008; Keller et al., 1993; Keller, 1995; Watanabe et al., 2005). These approaches resulted in the generation of endodermal, mesodermal and ectodermal cells that could then be patterned along anterior-posterior or dorsal-ventral axes to adopt early organ fate. However, early efforts at directed differentiation of human PSCs were largely made using monolayer cultures, resulting in cells that expressed markers of specific germ layers but which lacked tissue architecture (D'Amour et al., 2006; Osakada et al., 2009). Initial efforts to generate three-dimensional (3D) structures largely used aggregation and spontaneous differentiation, resulting in poorly organized mixtures of tissue structures (Itskovitz-Eldor et al., 2000). More recently, there has been significant progress in the generation of 3D organized tissues – referred to as organoids – in vitro from PSCs, and it is now possible to generate tissues that represent a broad range of organs, including the brain, kidney and intestine among many others. In this review, we highlight the essential role that developmental biology has played in directing the differentiation of PSCs into 3D human organoids in vitro. We highlight examples where signaling pathways that govern germ layer formation, patterning, organ induction and tissue morphogenesis guide the formation of organoids, much as they do in vivo. We also discuss examples in which laboratories have modeled the normal tissue-tissue interactions that occur between multiple germ layers in vivo to engineer organoids with increasing complexity in vitro. Lastly, we describe several potential applications of organoids as a model system, and discuss the challenges and limitations that must be overcome in the next phase of organoid engineering. We note that organoids can also be generated from adult tissue stem cells; however, since this approach does not invoke developmental principles to the same extent as PSC-derived organoids, adult tissue-derived organoids will not be discussed here.
Patterning and morphogenesis are governed by common developmental pathways
Standard PSC culture conditions allow for unlimited self-renewal, while at the same time maintaining pluripotency. However, exposing PSCs to specific combinations and concentrations of growth factors and signals, as required by the developing embryo, rapidly triggers PSC differentiation, morphogenesis, and the formation of organoids with cellular and tissue similarities to organs of all three germ layers. Studies performed in model organisms have identified that Wnt, FGF, retinoic acid (RA) and TGFβ/BMP are the main pathways that govern germ layer formation, patterning and the induction of organ primordia. One of the most profound questions in developmental biology is how such a small number of signaling pathways can act to direct the formation of such a broad array of diverse tissues. The use of these pathways to regulate organoid formation has helped to address this question, and identified that the timing, dose and combination of factors used can result in vastly different outcomes. These basic principles underlie the successful generation of human organoids that resemble the brain (Lancaster et al., 2013), eyes (Nakano et al., 2012), kidney (Takasato et al., 2014), lung (Dye et al., 2015), stomach (McCracken et al., 2014) and intestine (Spence et al., 2011).
During gastrulation, the migration of epiblast cells through the primitive streak segregates the mesoderm and endoderm from the ectoderm, with Nodal, a member of the TGFβ superfamily, required for mesoderm and endoderm formation (Arnold and Robertson, 2009; Zorn and Wells, 2009). Pioneering studies by Gordon Keller's group modeled the formation of the primitive streak and resulting mesoderm and endoderm germ layers using activin A, a Nodal mimetic, in murine ESC cultures (Gadue et al., 2006; Kattman et al., 2011; Kubo et al., 2004). Similarly, human PSCs can be directed to adopt a mesendodermal fate by exposure to activin A, with longer exposure to high levels of activin A capable of driving the formation of definitive endoderm (D'Amour et al., 2005).
While Nodal and Wnt signaling are essential for gastrulation to occur in the posterior epiblast of mice (Zorn and Wells, 2009), repression of these pathways in the anterior epiblast is essential for neuroectoderm formation, and this concept has proven true for the neural induction of PSCs by culture in minimal media with small-molecule inhibition (Chambers et al., 2009). Yoshiki Sasai's group established a method for the efficient differentiation of neural progenitors in vitro by dissociating PSCs to dilute all endogenous signals, allowing them to reaggregate in suspension, and culturing the resulting aggregates in minimal media under Wnt inhibition (Eiraku et al., 2008; Watanabe et al., 2005, 2007; Wataya et al., 2008). Transfer of the resulting aggregates into 3D suspension in Matrigel and additional culture in an orbital shaker resulted in the formation of cerebral organoids with layers of differentiated neurons (Lancaster et al., 2013). Alternatively, culturing free-floating neural aggregates in a small amount of dissolved Matrigel promotes the formation of a rigid neuroepithelium that self-organizes into optic cups that are capable of forming retinal epithelium (Nakano et al., 2012). A defining feature of directing the differentiation of PSCs into neural tissues is the absence of inductive signals, but once neural identity is established the neuroepithelium requires patterning factors to form organ-like structures, with defined cerebral regional domains arising spontaneously in the presence of RA (Lancaster et al., 2013), and improved retinal epithelial differentiation in the presence of fetal bovine serum, sonic hedgehog and Wnt (Nakano et al., 2012). In monolayer PSC-derived neural cultures, temporal modulation of Wnt, FGF and RA signals patterns neuromesodermal progenitors along the rostral-caudal axis (Lippmann et al., 2015), suggesting that brain organoids might be amenable to precise patterning to generate specific cerebral domains.
After the establishment of the three germ layers, endoderm and mesoderm are patterned along the anterior-posterior axis of the embryo and this is controlled by spatial and temporal gradients of Wnt, FGF, RA and TGFβ/BMP. These four signaling pathways promote posterior endoderm identity via the transcription factor Cdx2, an evolutionarily conserved posterior determinant (Zorn and Wells, 2009), while inhibition of BMP signaling is required for anterior endoderm patterning (Fausett et al., 2014). Manipulation of these pathways has proven effective in patterning PSC-derived definitive endoderm. Activation of Wnt and FGF signaling promotes the expression of CDX2 and results in commitment to mid/hindgut fate (Spence et al., 2011). Inhibition of BMP signaling, in conjunction with FGF and Wnt activation, is required to repress Cdx2 and the posterior program and instead promotes the formation of Sox2-expressing foregut endoderm (McCracken et al., 2014). Addition of RA patterns foregut posteriorly and results in gastric spheroids that will form antral organoids (McCracken et al., 2014), while continued activation of Wnt signaling in gastric organoids promotes fundic/corpus organoids that produce acid and digestive enzymes (McCracken et al., 2017). Inhibition of TGFβ and BMP in foregut endoderm results in anterior foregut that is competent to give rise to respiratory lineages (Dye et al., 2015). In mesoderm, modeling the timing of the migration of presomitic mesoderm through the primitive streak by the sequential activation of Wnt then FGF signaling results in anterior-patterned and posterior-patterned intermediate mesoderm that give rise to ureteric epithelium and metanephric mesenchyme, respectively (Taguchi et al., 2014; Takasato et al., 2014, 2015). RA is involved in promoting ureteric epithelial identity, whereas inhibition of RA signaling promotes metanephric mesenchyme (Takasato et al., 2015).
In addition to their role as posteriorizing factors, Wnt and FGF signals simultaneously promote the morphogenesis of 2D endoderm culture into 3D spheroids that are similar to the posterior gut tube (Dye et al., 2015; McCracken et al., 2014; Spence et al., 2011). This is also true for mesoderm, where prolonged exposure to Wnt and FGF promotes 3D growth of the developing kidney organoid (Takasato et al., 2014, 2015), and for ectoderm, where addition of a Wnt agonist promotes the morphogenesis of retinal pigmented epithelium (Nakano et al., 2012). Similar to the neuroepithelium, once patterned, the growth and development of mesodermal and endodermal organoids are supported by tissue-specific factors. Sustained FGF signaling is required for nephrogenesis in kidney organoids (Takasato et al., 2015), epidermal growth factor (EGF) is required to maintain gastric (McCracken et al., 2014) and intestinal (Spence et al., 2011) organoids, and FGF and sonic hedgehog are required to promote in vitro lung epithelial development (Dye et al., 2015).
Interactions between multiple germ layers improve in vitro complexity
In the embryo, interactions between endoderm and mesoderm are crucial for early germ layer patterning, and at later stages of endodermal organ development epithelial-mesenchymal interactions are essential for cell differentiation and tissue morphogenesis. The successful generation of lung, gastric and intestinal organoids has depended on the presence of low levels of mesoderm in activin A-treated cultures. The resulting mesoderm surrounds the epithelium of developing organoids and differentiates into fibroblasts and smooth muscle (Dye et al., 2015; McCracken et al., 2014; Spence et al., 2011). In vivo, endodermal organ primordia are also surrounded by mesenchyme, which is believed to help drive tissue morphogenesis (Zorn and Wells, 2009). The epithelium of PSC-derived lung, gastric and intestinal organoids undergoes remarkable morphogenesis and forms villus-like structures (intestine) and glandular structures (stomach). It seems reasonable to speculate that the mesenchyme might help drive this phenomenon via epithelial-mesenchymal interactions in vitro.
The approach to generate 3D liver organoids from PSCs also utilizes mesodermal cell types to trigger the formation of liver bud-like structures. The liver is a highly vascularized tissue, and morphogenesis of the developing liver bud is dependent on endothelial cells in vivo, with failure of liver bud organogenesis in mice that lack vascular endothelial cells (Matsumoto et al., 2001). Recently, Takebe and colleagues used an approach whereby PSCs were directed to differentiate into hepatic endoderm, at which point human mesenchymal stem cells and vascular endothelial cells were added to the cultures. This resulted in the generation of a self-organizing 3D liver bud in vitro (Takebe et al., 2013) that contained clearly defined vascular capillaries capable of blood flow when transplanted into mice. This approach was used to generate and achieve vascularization of other organ bud-like structures when transplanted in vivo, including the pancreas (Takebe et al., 2015).
In the embryo, developing endodermal and mesodermal organs also become innervated, and these peripheral nerves are required for function of the adult organ (Aven and Ai, 2013; Hatch and Mukouyama, 2015; Pichel et al., 1996). For example, the intestine contains 500 million neurons, and these all derive from neural crest cells that migrate into the gut mesenchyme shortly after gut tube formation. Development of these enteric neurons occurs in parallel with that of the epithelium and mesenchymal cell types. Although PSC-derived human intestinal organoids do not contain enteric nerves, a recent study engineered neurons into organoids by experimentally combining PSC-derived neural crest cells with mid/hindgut spheroids, resulting in self-organizing human intestinal organoids with a functional enteric nervous system (Workman et al., 2016). This system was used to study Hirschsprung's disease, a gut motility disorder resulting from defective enteric neuron development. A similar approach could be used to generate functionally innervated stomach organoids for studies of digestion or for innervation of the kidney to study networks essential for maintaining water and sodium homeostasis. Moreover, incorporation of neurons into pancreatic organoids might enhance the maturity of cell types, since there is evidence that signaling cross-talk between neurons and pancreatic endocrine cells is important for beta cell maturation (Nekrep et al., 2008), and the combination of neural crest cells with isolated human islets improves engraftment (Grapensparr et al., 2015).
In the future, engineering organoids to contain supporting cell types such as blood vessels and neurons might become standard. For example, the embryonic brain begins to develop in the absence of vasculature (Vasudevan et al., 2008), but later brain development requires blood vessels to provide a neural progenitor niche (Javaherian and Kriegstein, 2009; Lange et al., 2016). Cerebral organoids, which lack any mesenchymal input, recapitulate the structure, complexity and transcriptional program of first-trimester fetal brain (Kelava and Lancaster, 2016; Lancaster et al., 2013); however, further maturation of brain organoids is likely to require the incorporation of vascular components to support neuronal differentiation. Microglia, which arise via primitive myelopoiesis, are also essential in neuronal maturation and development of the central nervous system. A recent report described the generation of microglia-like cells from human PSCs that integrate into a 3D organotypic neuroglial environment and respond to cellular damage (Muffat et al., 2016). Incorporation of PSC-derived microglia into cerebral organoids will provide an unprecedented opportunity to study the interactions between primitive myeloid-derived cells and early brain development. In addition to microglia-like cells, hematopoietic progenitor cells have also been generated from human PSCs (Choi et al., 2009a,b), although the generation of mature hematopoietic cells, including blood cells, remains elusive. Further improvements in the maturation of functional myeloid-derived cells and their incorporation into organoids will prove instrumental in understanding innate immunity and the epithelial response to pathogens and tissue-tissue interactions in early human development. Recapitulating in vivo cellular interactions that occur during organogenesis by experimentally combining components of multiple germ layers to generate innervated and vascularized organoids is the next step in tissue engineering and will be essential for regenerative medicine.
Organoids: new models to study old diseases
Although animal models of development and disease are still essential in biomedical research, organoids provide unprecedented access to understanding aspects of developmental biology that are unique to humans. They also provide the opportunity to model human diseases in a more complex way than previously possible, especially those that affect the developing fetus. For example, cerebral organoids provide a unique opportunity to study not only brain development, but also specific neurological disease processes, such as the microcephaly that is secondary to infection with Zika virus (Dang et al., 2016; Garcez et al., 2016; Qian et al., 2016, 2017). Gastrointestinal organoids have also proven useful in investigating other diseases that are difficult to study in animal models. Intestinal organoids infected with Clostridium difficile (Leslie et al., 2015) or gastric organoids infected with Helicobacter pylori (Bertaux-Skeirik et al., 2015; McCracken et al., 2014) have been essential in understanding some of the earliest processes in the epithelial response to pathogens. Additionally, with the advent of CRISPR/Cas9 gene editing, disease modeling, mutation correction and personalized medicine are now possible in patient-specific iPSC-derived organoids (Hockemeyer and Jaenisch, 2016). Patient-specific iPSC-derived cerebral organoids have been generated for the study of microcephaly (Lancaster et al., 2013), and CRISPR/Cas9 gene editing has enabled the study of polycystic kidney disease within kidney organoids (Freedman et al., 2015). In the future, patient-specific iPSC-derived organoids might be used to predict individualized drug efficacy and epithelial response, as has recently been shown for patients with cystic fibrosis using adult tissue-derived organoids (Saini, 2016).
Conclusions
The application of fundamental principles of developmental biology to the directed differentiation of human PSCs has driven the recent development of human organoid systems. By applying additional developmental principles, highly complex and functional organoids are being developed that contain components of all three germ layers. Access to human organoids, whether derived from ESCs or patient-specific iPSCs, is shaping the future of biomedical research.
The two greatest challenges facing the field are scalability and improving the maturity of organoids in vitro. There has been recent success in growing brain organoids using miniature, multiwell spinning bioreactors, which has the potential for large-scale organoid generation and high-throughput drug screening (Qian et al., 2016). Building complexity by combining PSC-derived tissues is a step toward more functionally mature organoids, although these still more closely resemble fetal than adult tissue. For advances in organ replacement therapy, generating PSC-derived organoids that can fully mature to attain adult structure and function in vitro is essential. Growth, development and functional improvements are needed before organoid-based transplants could be used therapeutically. However, the in vivo environment itself is one key route to improving the functionality of intestinal organoids (Watson et al., 2014), so momentum is building toward transplantable tissue engineered organs. Considering the pace at which the PSC-derived organoid field has advanced over the past five years, the range of possible future applications of organoids in the study of development and disease and in regenerative medicine is arguably limitless.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The authors are supported by National Institutes of Health grants (R01DK092456, U18EB021780, U01DK103117, U19AI116491 to J.M.W.) and a fellowship from the American Diabetes Association (1-17-PDF-102 to H.A.M.). Deposited in PMC for release after 12 months.
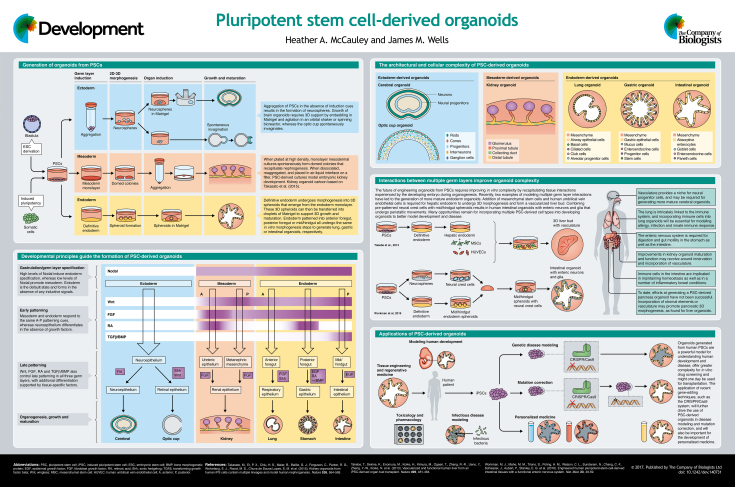
Development at a Glance
A high-resolution version of the poster is available for downloading in the online version of this article at http://dev.biologists.org/content/144/6/958/F1.poster.jpg
References
- Arnold S. J. and Robertson E. J. (2009). Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 10, 91-103. 10.1038/nrm2618 [DOI] [PubMed] [Google Scholar]
- Aven L. and Ai X. (2013). Mechanisms of respiratory innervation during embryonic development. Organogenesis 9, 194-198. 10.4161/org.24842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertaux-Skeirik N., Feng R., Schumacher M. A., Li J., Mahe M. M., Engevik A. C., Javier J. E., Peek R. M. Jr, Ottemann K., Orian-Rousseau V. et al. (2015). CD44 plays a functional role in Helicobacter pylori-induced epithelial cell proliferation. PLoS Pathog. 11, e1004663 10.1371/journal.ppat.1004663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S. M., Fasano C. A., Papapetrou E. P., Tomishima M., Sadelain M. and Studer L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275-280. 10.1038/nbt.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.-D., Vodyanik M. A. and Slukvin I. I. (2009a). Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin-CD34+CD43+CD45+ progenitors. J. Clin. Invest. 119, 2818-2829. 10.1172/JCI38591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.-D., Yu J., Smuga-Otto K., Salvagiotto G., Rehrauer W., Vodyanik M., Thomson J. and Slukvin I. (2009b). Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27, 559-567. 10.1002/stem.20080922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour K. A., Agulnick A. D., Eliazer S., Kelly O. G., Kroon E. and Baetge E. E. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534-1541. 10.1038/nbt1163 [DOI] [PubMed] [Google Scholar]
- D'Amour K. A., Bang A. G., Eliazer S., Kelly O. G., Agulnick A. D., Smart N. G., Moorman M. A., Kroon E., Carpenter M. K. and Baetge E. E. (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392-1401. 10.1038/nbt1259 [DOI] [PubMed] [Google Scholar]
- Dang J., Tiwari S. K., Lichinchi G., Qin Y., Patil V. S., Eroshkin A. M. and Rana T. M. (2016). Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19, 258-265. 10.1016/j.stem.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbaillets I., Ziegler U., Groscurth P. and Gassmann M. (2000). Embryoid bodies: an in vitro model of mouse embryogenesis. Exp. Physiol. 85, 645-651. 10.1111/j.1469-445X.2000.02104.x [DOI] [PubMed] [Google Scholar]
- Dye B. R., Hill D. R., Ferguson M. A. H., Tsai Y.-H., Nagy M. S., Dyal R., Wells J. M., Mayhew C. N., Nattiv R., Klein O. D. et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, e05098 10.7554/elife.05098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K. and Sasai Y. (2008). Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519-532. 10.1016/j.stem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T. and Sasai Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51-56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Fausett S. R., Brunet L. J. and Klingensmith J. (2014). BMP antagonism by Noggin is required in presumptive notochord cells for mammalian foregut morphogenesis. Dev. Biol. 391, 111-124. 10.1016/j.ydbio.2014.02.008 [DOI] [PubMed] [Google Scholar]
- Freedman B. S., Brooks C. R., Lam A. Q., Fu H., Morizane R., Agrawal V., Saad A. F., Li M. K., Hughes M. R., Werff R. V. et al. (2015). Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun. 6, 8715 10.1038/ncomms9715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadue P., Huber T. L., Paddison P. J. and Keller G. M. (2006). Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. USA 103, 16806-16811. 10.1073/pnas.0603916103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez P. P., Loiola E. C., Madeiro da Costa R., Higa L. M., Trindade P., Delvecchio R., Nascimento J. M., Brindeiro R., Tanuri A. and Rehen S. K. (2016). Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816-818. 10.1126/science.aaf6116 [DOI] [PubMed] [Google Scholar]
- Grapensparr L., Vasylovska S., Li Z., Olerud J., Jansson L., Kozlova E. and Carlsson P.-O. (2015). Co-transplantation of human pancreatic islets with post-migratory neural crest stem cells increases β-cell proliferation and vascular and neural regrowth. J. Clin. Endocrinol. Metab. 100, E583-E590. 10.1210/jc.2014-4070 [DOI] [PubMed] [Google Scholar]
- Hatch J. and Mukouyama Y.-S. (2015). Spatiotemporal mapping of vascularization and innervation in the fetal murine intestine. Dev. Dyn. 244, 56-68. 10.1002/dvdy.24178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D. and Jaenisch R. (2016). Induced pluripotent stem cells meet genome editing. Cell Stem Cell 18, 573-586. 10.1016/j.stem.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., Soreq H. and Benvenisty N. (2000). Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 6, 88-95. [PMC free article] [PubMed] [Google Scholar]
- Javaherian A. and Kriegstein A. (2009). A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb. Cortex 19 Suppl. 1, i70-i77. 10.1093/cercor/bhp029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman S. J., Witty A. D., Gagliardi M., Dubois N. C., Niapour M., Hotta A., Ellis J. and Keller G. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228-240. 10.1016/j.stem.2010.12.008 [DOI] [PubMed] [Google Scholar]
- Kelava I. and Lancaster M. A. (2016).Dishing out mini-brains: current progress and future prospects in brain organoid research. Dev. Biol. 420, 199-209. 10.1016/j.ydbio.2016.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. M. (1995). In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 7, 862-869. 10.1016/0955-0674(95)80071-9 [DOI] [PubMed] [Google Scholar]
- Keller G., Kennedy M., Papayannopoulou T. and Wiles M. V. (1993). Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol. Cell. Biol. 13, 473-486. 10.1128/MCB.13.1.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A., Shinozaki K., Shannon J. M., Kouskoff V., Kennedy M., Woo S., Fehling H. J. and Keller G. (2004). Development of definitive endoderm from embryonic stem cells in culture. Development 131, 1651-1662. 10.1242/dev.01044 [DOI] [PubMed] [Google Scholar]
- Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., Homfray T., Penninger J. M., Jackson A. P. and Knoblich J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373-379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Turrero Garcia M., Decimo I., Bifari F., Eelen G., Quaegebeur A., Boon R., Zhao H., Boeckx B., Chang J. et al. (2016). Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis. EMBO J. 35, 924-941. 10.15252/embj.201592372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie J. L., Huang S., Opp J. S., Nagy M. S., Kobayashi M., Young V. B. and Spence J. R. (2015). Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 83, 138-145. 10.1128/IAI.02561-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann E. S., Williams C. E., Ruhl D. A., Estevez-Silva M. C., Chapman E. R., Coon J. J. and Ashton R. S. (2015). Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Rep. 4, 632-644. 10.1016/j.stemcr.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Yoshitomi H., Rossant J. and Zaret K. S. (2001). Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559-563. 10.1126/science.1063889 [DOI] [PubMed] [Google Scholar]
- McCracken K. W., Catá E. M., Crawford C. M., Sinagoga K. L., Schumacher M., Rockich B. E., Tsai Y.-H., Mayhew C. N., Spence J. R., Zavros Y. et al. (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400-404. 10.1038/nature13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken K. W., Aihara E., Martin B., Crawford C. M., Broda T., Treguier J., Zhang X., Shannon J. M., Montrose M. H. and Wells J. M. (2017). Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature 541, 182-187. 10.1038/nature21021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J., Li Y., Yuan B., Mitalipova M., Omer A., Corcoran S., Bakiasi G., Tsai L.-H., Aubourg P., Ransohoff R. M. et al. (2016). Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 22, 1358-1367. 10.1038/nm.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M. and Sasai Y. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771-785. 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Nekrep N., Wang J., Miyatsuka T. and German M. S. (2008). Signals from the neural crest regulate beta-cell mass in the pancreas. Development 135, 2151-2160. 10.1242/dev.015859 [DOI] [PubMed] [Google Scholar]
- Osakada F., Jin Z.-B., Hirami Y., Ikeda H., Danjyo T., Watanabe K., Sasai Y. and Takahashi M. (2009). In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J. Cell Sci. 122, 3169-3179. 10.1242/jcs.050393 [DOI] [PubMed] [Google Scholar]
- Pichel J. G., Shen L., Sheng H. Z., Granholm A.-C., Drago J., Grinberg A., Lee E. J., Huang S. P., Saarma M., Hoffer B. J. et al. (1996). Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382, 73-76. 10.1038/382073a0 [DOI] [PubMed] [Google Scholar]
- Qian X., Nguyen H. N., Song M. M., Hadiono C., Ogden S. C., Hammack C., Yao B., Hamersky G. R., Jacob F., Zhong C. et al. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238-1254. 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Nguyen H. N., Jacob F., Song H. and Ming G.-l. (2017). Using brain organoids to understand Zika virus-induced microcephaly. Development 144, 952-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini A. (2016). Cystic fibrosis patients benefit from mini guts. Cell Stem Cell 19, 425-427. 10.1016/j.stem.2016.09.001 [DOI] [Google Scholar]
- Spence J. R., Mayhew C. N., Rankin S. A., Kuhar M. F., Vallance J. E., Tolle K., Hoskins E. E., Kalinichenko V. V., Wells S. I., Zorn A. M. et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105-109. 10.1038/nature09691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A., Kaku Y., Ohmori T., Sharmin S., Ogawa M., Sasaki H. and Nishinakamura R. (2014). Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53-67. 10.1016/j.stem.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861-872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Takasato M., Er P. X., Becroft M., Vanslambrouck J. M., Stanley E. G., Elefanty A. G. and Little M. H. (2014). Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat. Cell Biol. 16, 118-126. 10.1038/ncb2894 [DOI] [PubMed] [Google Scholar]
- Takasato M., Er P. X., Chiu H. S., Maier B., Baillie G. J., Ferguson C., Parton R. G., Wolvetang E. J., Roost M. S., Chuva de Sousa Lopes S. M. et al. (2015). Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564-568. 10.1038/nature15695 [DOI] [PubMed] [Google Scholar]
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.-R., Ueno Y., Zheng Y.-W., Koike N. et al. (2013). Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481-484. 10.1038/nature12271 [DOI] [PubMed] [Google Scholar]
- Takebe T., Enomura M., Yoshizawa E., Kimura M., Koike H., Ueno Y., Matsuzaki T., Yamazaki T., Toyohara T., Osafune K. et al. (2015). Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16, 556-565. 10.1016/j.stem.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S. and Jones J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145-1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Vasudevan A., Long J. E., Crandall J. E., Rubenstein J. L. R. and Bhide P. G. (2008). Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat. Neurosci. 11, 429-439. 10.1038/nn2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Kamiya D., Nishiyama A., Katayama T., Nozaki S., Kawasaki H., Watanabe Y., Mizuseki K. and Sasai Y. (2005). Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288-296. 10.1038/nn1402 [DOI] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J. B., Nishikawa S., Nishikawa S., Muguruma K. et al. (2007). A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681-686. 10.1038/nbt1310 [DOI] [PubMed] [Google Scholar]
- Wataya T., Ando S., Muguruma K., Ikeda H., Watanabe K., Eiraku M., Kawada M., Takahashi J., Hashimoto N. and Sasai Y. (2008). Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Natl. Acad. Sci. USA 105, 11796-11801. 10.1073/pnas.0803078105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. L., Mahe M. M., Múnera J., Howell J. C., Sundaram N., Poling H. M., Schweitzer J. I., Vallance J. E., Mayhew C. N., Sun Y. et al. (2014). An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 20, 1310-1314. 10.1038/nm.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman M. J., Mahe M. M., Trisno S., Poling H. M., Watson C. L., Sundaram N., Chang C.-F., Schiesser J., Aubert P., Stanley E. G. et al. (2016). Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat. Med. 23, 49-59. 10.1038/nm.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn A. M. and Wells J. M. (2009). Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221-251. 10.1146/annurev.cellbio.042308.113344 [DOI] [PMC free article] [PubMed] [Google Scholar]