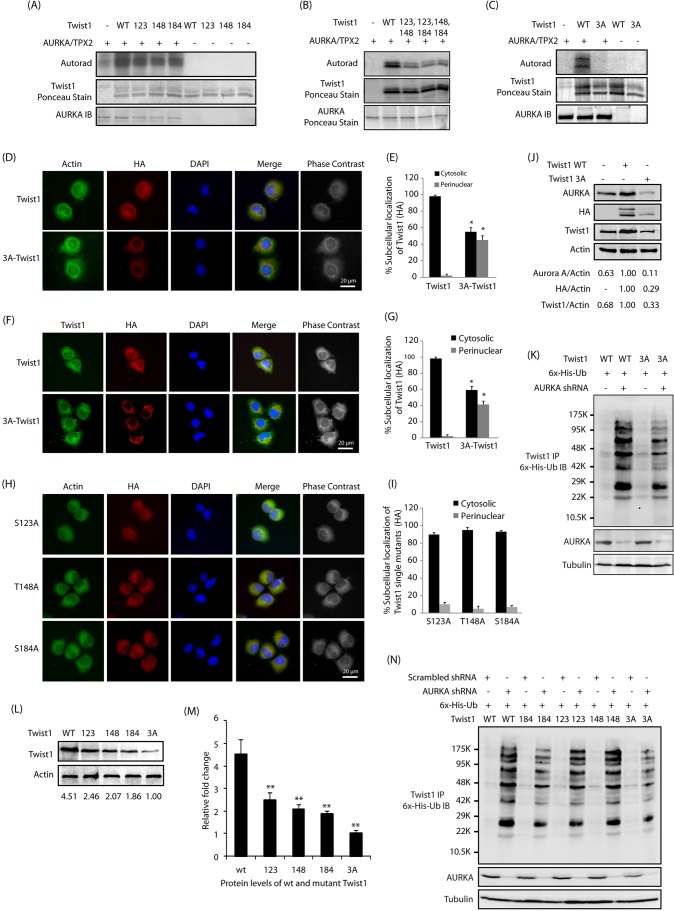
Fig. 4.
AURKA-mediated phosphorylation of Twist1 at S123, T148 and S184 regulates its protein stability and subcellular localization. (A) AURKA phosphorylates Twist1 at S123, T148 and S184. Phospho-dead single mutants of Twist1 (S123A, T148A and S184A, denoted 123, 148 and 184) were subjected to an in vitro kinase assay using AURKA–TPX2. IB, immunoblot; WT, wild-type. (B) Phosphorylation of Twist1 double mutants by AURKA. (C) S123, T148 and S184 are the only AURKA sites on Twist1. (D) AURKA regulates the subcellular localization of Twist1 via phosphorylation. BxPC3 cells stably expressing HA-tagged wild-type and 3A-Twist1 were fixed and immunostained with anti-actin and anti-HA antibody. More than 100 cells were analyzed from multiple random frames. Representative data are shown. (E) Histogram showing the percentage of BxPC3 cells as in D displaying cytosolic and perinuclear localization of ectopically expressed HA-tagged Twist1. Values shown are mean±s.e.m. of three independent experiments. *P<0.05 with respect to controls (two-way ANOVA). (F) Differential localization of wild-type and 3A Twist1 mutant in BxPC3 cells. (G) Histogram showing the percentage of BxPC3 cells expressing wild-type Twist1 and 3A-Twist1 showing cytosolic and perinuclear localization of Twist1. Values shown are mean±s.e.m. of three independent experiments. *P<0.05 with respect to controls (two-way ANOVA). (H) AURKA regulates the subcellular localization of Twist1 via phosphorylation of all three sites. Representative data are shown. (I) Histogram showing the percentage of single mutant Twist1-BxPC3 cells displaying cytosolic and perinuclear localization. Values shown are mean±s.e.m. of three independent experiments. *P<0.05 with respect to controls (two-way ANOVA). (J) AURKA promotes Twist1 levels through phosphorylation. (K) AURKA inhibits Twist1 ubiquitylation by phosphorylating S123, T148 and S184 sites. AURKA depletion in WT-Twist1-expressing cells causes higher levels of ubiquitylation than its depletion in 3A-Twist1-expressing cells. (L) AURKA increases Twist1 levels through phosphorylation at S123, T148 and S184. The ratio of Twist1 to actin from a representative experiment is given below the blots. (M) Graphical representation of Twist1 levels in BxPC3 cells expressing either wild-type Twist1, S123A Twist1, T148A Twist1, S184A Twist1 or 3A-Twist1. Mean±s.e.m. values of wild-type and mutant Twist1 levels from three independent experiments are depicted in the graph. **P<0.01 compared to control (one-way ANOVA). (N) AURKA inhibits Twist1 ubiquitylation by phosphorylating S123, T148 and S184 sites. Each experiment was done at least three independent times. Representative data are shown.